The Exocytosis-regulatory protein synaptotagmin VII mediates cell invasion by Trypanosoma cruzi - PubMed (original) (raw)
The Exocytosis-regulatory protein synaptotagmin VII mediates cell invasion by Trypanosoma cruzi
E V Caler et al. J Exp Med. 2001.
Abstract
The intracellular protozoan parasite Trypanosoma cruzi causes Chagas' disease, which affects millions of people in Latin America. T. cruzi enters a large number of cell types by an unusual mechanism that involves Ca(2+)-triggered fusion of lysosomes with the plasma membrane. Here we show that synaptotagmin VII (Syt VII), a ubiquitously expressed synaptotagmin isoform that regulates exocytosis of lysosomes, is localized on the membranes of intracellular vacuoles containing T. cruzi. Antibodies against the C(2)A domain of Syt VII or recombinant peptides including this domain inhibit cell entry by T. cruzi, but not by Toxoplasma gondii or Salmonella typhimurium. The C(2)A domains of other ubiquitously expressed synaptotagmin isoforms have no effect on T. cruzi invasion, and mutation of critical residues on Syt VII C(2)A abolish its inhibitory activity. These findings indicate that T. cruzi exploits the Syt VII-dependent, Ca(2+)-regulated lysosomal exocytic pathway for invading host cells.
Figures
Figure 1
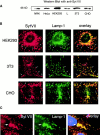
Syt VII is localized on the membranes of lysosomes and of _T. cruzi_–containing intracellular vacuoles. (A) Detection of Syt VII on extracts of different cell types by Western blot using antibodies specific for the unique NH2-terminal Syt VII ectodomain. (B) Double immunofluorescence showing colocalization of Syt VII (red) and the lysosomal glycoprotein Lamp-1 (green) in HEK-293, 3T3, and CHO cells. Arrows point to examples of compartments double positive for Syt VII and Lamp-1. (C) Double immunofluorescence showing colocalization of Syt VII (red) and Lamp-1 (green) on the membranes of intracellular vacuoles containing T. cruzi (arrows). DAPI staining of host cell and parasite DNA is shown in blue.
Figure 2
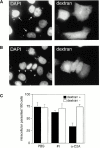
Antibodies against the Syt VII C2A domain inhibit cell invasion by T. cruzi. (A) DAPI DNA stain (left) and Texas Red–dextran (right) fluorescent images of CHO cells loaded with preimmune rabbit IgG. Arrows point to infected cells previously loaded with dextran and IgG. (B) Same as A, except that the cells were loaded with affinity-purified antibodies against the Syt VII C2A domain. Arrowheads point to uninfected cells previously loaded with dextran and IgG. (C) Quantitation of the number of intracellular trypomastigotes found in cells loaded (black bars) or unloaded (white bars) with dextran, in the presence of PBS only or of preimmune (PI) or anti–Syt VII C2A antibodies (α-C2A). The data is expressed as the mean ± SD of triplicate infections.
Figure 3
Expression of the Syt VII C2A domain inhibits cell invasion by T. cruzi but not by T. gondii or S. typhimurium. (A) T. cruzi invasion of CHO cells untransfected (gray bars; unt) or transfected (white bars) with GFPv, Syt I C2A–GFP, and Syt VII C2A–GFP. (B) T. gondii invasion of CHO cells transfected with GFPv, Syt I C2A–GFP, and Syt VII C2A–GFP. (C) S. typhymurium invasion of CHO cells transfected with GFPv, Syt I C2A–GFP, and Syt VII C2A–GFP. The data is expressed as the mean ± SD of triplicate infections. The color images below panels A, B, and C illustrate the immunofluorescence detection assays used to distinguish intracellular and extracellular pathogens: GFP is shown in green, antibodies against T. cruzi, Toxoplasma, or Salmonella in red, and DAPI DNA stain in blue. Arrows point to extracellular organisms and arrowheads to intracellular ones.
Figure 4
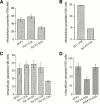
The inhibitory effect on T. cruzi invasion is specific for Syt VII and is abolished by mutation of critical C2A domain residues. The number of intracellular trypomastigotes was quantitated after infection of: (A) NRK cells transfected with GFPv (pEGFP-N2 vector alone), Syt I C2A–GFP, or Syt VII C2A–GFP; (B) NRK cells microinjected with his-tagged Syt I C2A or Syt VII C2A; (C) CHO cells transfected with GFPv or GFP-fused C2A domains of the synaptotagmin isoforms Syt I, VII, IX, or VIII; (D) CHO cells transfected with GFPv, Syt VII C2A–GFP, or mSyt VII C2A. The data is expressed as the mean ± SD of triplicate infections.
Similar articles
- Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts.
Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW. Martinez I, et al. J Cell Biol. 2000 Mar 20;148(6):1141-49. doi: 10.1083/jcb.148.6.1141. J Cell Biol. 2000. PMID: 10725327 Free PMC article. - Synaptotagmin VII is targeted to dense-core vesicles and regulates their Ca2+ -dependent exocytosis in PC12 cells.
Fukuda M, Kanno E, Satoh M, Saegusa C, Yamamoto A. Fukuda M, et al. J Biol Chem. 2004 Dec 10;279(50):52677-84. doi: 10.1074/jbc.M409241200. Epub 2004 Sep 28. J Biol Chem. 2004. PMID: 15456748 - Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes.
Reddy A, Caler EV, Andrews NW. Reddy A, et al. Cell. 2001 Jul 27;106(2):157-69. doi: 10.1016/s0092-8674(01)00421-4. Cell. 2001. PMID: 11511344 - Don't bother to knock--the cell invasion strategy of Trypanosoma cruzi.
Tan H, Andrews NW. Tan H, et al. Trends Parasitol. 2002 Oct;18(10):427-8. doi: 10.1016/s1471-4922(02)02368-1. Trends Parasitol. 2002. PMID: 12377585 Review. - Synaptotagmin regulates mast cell functions.
Baram D, Mekori YA, Sagi-Eisenberg R. Baram D, et al. Immunol Rev. 2001 Feb;179:25-34. doi: 10.1034/j.1600-065x.2001.790103.x. Immunol Rev. 2001. PMID: 11292024 Review.
Cited by
- Active penetration of Trypanosoma cruzi into host cells: historical considerations and current concepts.
de Souza W, de Carvalho TM. de Souza W, et al. Front Immunol. 2013 Jan 25;4:2. doi: 10.3389/fimmu.2013.00002. eCollection 2013. Front Immunol. 2013. PMID: 23355838 Free PMC article. - Lysosomes and the plasma membrane: trypanosomes reveal a secret relationship.
Andrews NW. Andrews NW. J Cell Biol. 2002 Aug 5;158(3):389-94. doi: 10.1083/jcb.200205110. Epub 2002 Jul 29. J Cell Biol. 2002. PMID: 12147679 Free PMC article. Review. - Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes.
Czibener C, Sherer NM, Becker SM, Pypaert M, Hui E, Chapman ER, Mothes W, Andrews NW. Czibener C, et al. J Cell Biol. 2006 Sep 25;174(7):997-1007. doi: 10.1083/jcb.200605004. Epub 2006 Sep 18. J Cell Biol. 2006. PMID: 16982801 Free PMC article. - Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice.
Chakrabarti S, Kobayashi KS, Flavell RA, Marks CB, Miyake K, Liston DR, Fowler KT, Gorelick FS, Andrews NW. Chakrabarti S, et al. J Cell Biol. 2003 Aug 18;162(4):543-9. doi: 10.1083/jcb.200305131. J Cell Biol. 2003. PMID: 12925704 Free PMC article. - Molecular and cellular mechanisms involved in the Trypanosoma cruzi/host cell interplay.
Romano PS, Cueto JA, Casassa AF, Vanrell MC, Gottlieb RA, Colombo MI. Romano PS, et al. IUBMB Life. 2012 May;64(5):387-96. doi: 10.1002/iub.1019. Epub 2012 Mar 27. IUBMB Life. 2012. PMID: 22454195 Free PMC article. Review.
References
- Tardieux I., Webster P., Ravesloot J., Boron W., Lunn J.A., Heuser J.E., Andrews N.W. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell. 1992;71:1117–1130. - PubMed
- Dorta M.L., Ferreira A.T., Oshiro M.E.M., Yoshida N. Ca2+ signal induced by Trypanosoma cruzi metacyclic trypomastigote surface molecules implicated in mammalian cell invasion. Mol. Biochem. Parasitol. 1995;73:285–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous