The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment - PubMed (original) (raw)
The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment
E S Baekkevold et al. J Exp Med. 2001.
Abstract
Lymphocyte homing to secondary lymphoid tissue is defined by a multistep sequence of interactions between lymphocytes and endothelial cells in high endothelial venules (HEVs). After initial selectin-mediated tethering and rolling, firm adhesion of lymphocytes requires rapid upregulation of lymphocyte integrin adhesiveness. This step is mediated in part by the HEV-derived chemokine SLC (secondary lymphoid-tissue chemokine, or CCL21) that binds to the CC chemokine receptor (CCR)7 on lymphocytes. However, the CC chemokine ELC (Epstein-Barr virus-induced molecule 1 ligand chemokine, or CCL19) shares the same receptor, and ELC transcripts have been observed in the T cell areas of lymphoid organs. Here, we show that perivascular ELC is transcytosed to the luminal surfaces of HEVs and enables efficient T cell homing to lymph nodes. In situ hybridization on sections of human tonsil showed no ELC mRNA in HEVs, but immunostaining revealed ELC protein in cytoplasmic vesicles of HEV cells. Furthermore, ELC injected into the footpads of mice entered the draining lymph nodes and was presented by HEVs. Finally, intracutaneous injections of ELC in mice lacking functionally relevant ELC and SLC (plt/plt mice) restored T cell trafficking to draining lymph nodes as efficiently as SLC. We conclude that perivascular ELC is transcytosed to the luminal surfaces of HEVs and participates in CCR7-mediated triggering of lymphocyte arrest.
Figures
Figure 1
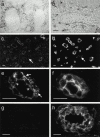
ELC protein is accumulated within HEVs, whereas ELC transcripts are found perivascularly. In situ hybridization with an RNA probe complementary to ELC mRNA. (a) Overview of tonsillar section showing positive cells (black) confined to T cell areas. (b) Higher magnification shows strong signals from perivascular cells partly surrounding the HEV (border delineated), whereas endothelial cells are negative. Single exposures of two-color immunofluorescence staining identifying ELC by means of MAB361 (c and e) and HEVs by means of mAb MECA-79 (d, f, and h). Most MECA79+ HEVs contain ELC, whereas MECA-79+ELC− vessels are also seen (c and d, arrows). MECA-79−ELC+ vessels are also found in the T cell zone (c and d, arrowheads). (e) HEV-associated ELC staining is distinctly luminal (arrowheads) and remarkably granular intra-cellularly (arrows), in contrast to the MECA-79 antigen distribution (f ). Staining with an irrelevant control mAb (g) produces no signal together with MECA-79 (h). Scale bars: b and e–h, 15 μm; c and d, 25 μm.
Figure 2
ELC protein is found in cytoplasmic vesicles of HEVs and at the HEV–lymphocyte interface. Silver-enhanced immunogold staining with goat anti–human ELC on sections of Unicryl-embedded tonsillar tissue. (a) Electron micrograph of interface between an HEV cell and lymphocyte shows vesicle-associated gold particles near the luminal plasma membrane of endothelial cells (arrows and high magnification insets). Note also membrane-proximal cytoplasmic vesicles of adjacent lymphocyte (arrowheads). (b and c) ELC labeling in intracellular vesicles (arrows and high magnification inset). Note absence of markers in the interendothelial cleft (b, arrowheads). (d) Staining with irrelevant control antibody. EC, endothelial cell; LC, lymphocyte; PC, pericyte. Scale bars: a and d, 500 nm; b and c, 200 nm.
Figure 3
HEVs possess binding sites for ELC. Autoradiography of tissue sections made after tonsillar organ culture with radioiodinated ELC. (a and b) Strong signals are predominately associated with HEV cells and the HEV lumen. In contrast, flat-walled vessels (c) show no signal. (d) Addition of excess cold ELC abolishes HEV-associated signals. Scale bars: 25 μm.
Figure 4
ELC injected into mice footpads is taken up by HEVs and presented at their luminal surfaces. 125I–ELC injected into BALB/c mice is detected by autoradiography in the afferent lymphatics (a, arrow) as well as in the subcapsular sinus (arrowheads) of draining lymph nodes after 90 min. In the T zone, ELC signals are predominately found in the HEVs (b, arrow) and lymphatic sinus (arrowheads). Higher magnification of areas outlined in c and e reveals radiolabel both inside HEV cells and on their luminal surfaces (d and f, arrows), as well as in the surrounding sinus (arrowheads). Injected 125I–MIP-1α is preferentially sequestered within the B zone, but HEV cells in the T zone are not labeled (g). Endothelial cells in contralateral lymph nodes contain no radiolabel (ELC-injected mouse shown in h). Scale bars: 25 μm.
Figure 5
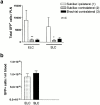
Intracutaneously injected ELC reconstitutes T cell homing to lymph nodes in plt/plt mice. plt/plt mice received intracutaneous injections of ELC, SLC (2.5 nmol), or PBS vehicle (50 μl) in the flank skin, followed by transfer of green fluorescent T cells (T–GFP) through the tail vein. (a) Total number of T–GFP cells homed to lymph nodes after 90 min. Significantly more cells homed to the ipsilateral than the contralateral lymph nodes. (b) There was no difference in the circulating level of T–GFP cells (*P < 0.005, **P < 0.003). Data are shown as mean ± SEM. n = 4 animals per chemokine.
Similar articles
- The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules.
Stein JV, Rot A, Luo Y, Narasimhaswamy M, Nakano H, Gunn MD, Matsuzawa A, Quackenbush EJ, Dorf ME, von Andrian UH. Stein JV, et al. J Exp Med. 2000 Jan 3;191(1):61-76. doi: 10.1084/jem.191.1.61. J Exp Med. 2000. PMID: 10620605 Free PMC article. - Augmented expression of secondary lymphoid tissue chemokine and EBI1 ligand chemokine in Crohn's disease.
Kawashima D, Oshitani N, Jinno Y, Watanabe K, Nakamura S, Higuchi K, Arakawa T. Kawashima D, et al. J Clin Pathol. 2005 Oct;58(10):1057-63. doi: 10.1136/jcp.2004.024828. J Clin Pathol. 2005. PMID: 16189151 Free PMC article. - CCR7-mediated physiological lymphocyte homing involves activation of a tyrosine kinase pathway.
Stein JV, Soriano SF, M'rini C, Nombela-Arrieta C, de Buitrago GG, Rodríguez-Frade JM, Mellado M, Girard JP, Martínez-A C. Stein JV, et al. Blood. 2003 Jan 1;101(1):38-44. doi: 10.1182/blood-2002-03-0841. Epub 2002 Jun 28. Blood. 2003. PMID: 12393730 - A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system.
Comerford I, Harata-Lee Y, Bunting MD, Gregor C, Kara EE, McColl SR. Comerford I, et al. Cytokine Growth Factor Rev. 2013 Jun;24(3):269-83. doi: 10.1016/j.cytogfr.2013.03.001. Epub 2013 Apr 12. Cytokine Growth Factor Rev. 2013. PMID: 23587803 Review. - Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues.
Ebert LM, Schaerli P, Moser B. Ebert LM, et al. Mol Immunol. 2005 May;42(7):799-809. doi: 10.1016/j.molimm.2004.06.040. Epub 2004 Nov 23. Mol Immunol. 2005. PMID: 15829268 Review.
Cited by
- Stromal cells control soluble material and cellular transport in lymph nodes.
Bajénoff M. Bajénoff M. Front Immunol. 2012 Sep 27;3:304. doi: 10.3389/fimmu.2012.00304. eCollection 2012. Front Immunol. 2012. PMID: 23060882 Free PMC article. - Locoregional Lymphatic Delivery Systems Using Nanoparticles and Hydrogels for Anticancer Immunotherapy.
Cho KJ, Cho YE, Kim J. Cho KJ, et al. Pharmaceutics. 2022 Dec 8;14(12):2752. doi: 10.3390/pharmaceutics14122752. Pharmaceutics. 2022. PMID: 36559246 Free PMC article. Review. - Chemokines and their receptors as biomarkers in esophageal cancer.
Goto M, Liu M. Goto M, et al. Esophagus. 2020 Apr;17(2):113-121. doi: 10.1007/s10388-019-00706-8. Epub 2019 Nov 26. Esophagus. 2020. PMID: 31773415 Review. - Leukocyte migration and graft-versus-host disease.
Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Wysocki CA, et al. Blood. 2005 Jun 1;105(11):4191-9. doi: 10.1182/blood-2004-12-4726. Epub 2005 Feb 8. Blood. 2005. PMID: 15701715 Free PMC article. Review. - Transcriptome analysis of chicken intraepithelial lymphocyte natural killer cells infected with very virulent infectious bursal disease virus.
Boo SY, Tan SW, Alitheen NB, Ho CL, Omar AR, Yeap SK. Boo SY, et al. Sci Rep. 2020 Oct 27;10(1):18348. doi: 10.1038/s41598-020-75340-x. Sci Rep. 2020. PMID: 33110122 Free PMC article.
References
- von Andrian U.H., Mackay C.R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. - PubMed
- Butcher E.C., Picker L.J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. - PubMed
- Girard J.P., Springer T.A. High endothelial venules (HEVs)specialized endothelium for lymphocyte migration. Immunol. Today. 1995;16:449–457. - PubMed
- Campbell J.J., Hedrick J., Zlotnik A., Siani M.A., Thompson D.A., Butcher E.C. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. - PubMed
- Cyster J.G. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL56946/HL/NHLBI NIH HHS/United States
- HL54936/HL/NHLBI NIH HHS/United States
- HL62524/HL/NHLBI NIH HHS/United States
- R01 HL054936/HL/NHLBI NIH HHS/United States
- P01 HL056949/HL/NHLBI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources