Biosynthesis of the glycolipid anchor in lipoteichoic acid of Staphylococcus aureus RN4220: role of YpfP, the diglucosyldiacylglycerol synthase - PubMed (original) (raw)
Biosynthesis of the glycolipid anchor in lipoteichoic acid of Staphylococcus aureus RN4220: role of YpfP, the diglucosyldiacylglycerol synthase
M Y Kiriukhin et al. J Bacteriol. 2001 Jun.
Abstract
In Staphylococcus aureus RN4220, lipoteichoic acid (LTA) is anchored in the membrane by a diglucosyldiacylglycerol moiety. The gene (ypfP) which encodes diglucosyldiacylglycerol synthase was recently cloned from Bacillus subtilis and expressed in Escherichia coli (P. Jorasch, F. P. Wolter, U. Zahringer, and E. Heinz, Mol. Microbiol. 29:419-430, 1998). To define the role of ypfP in this strain of S. aureus, a fragment of ypfP truncated from both ends was cloned into the thermosensitive replicon pVE6007 and used to inactivate ypfP. Chloramphenicol-resistant (ypfP::cat) clones did not synthesize the glycolipids monoglucosyldiacylglycerol and diglucosyldiacylglycerol. Thus, YpfP would appear to be the only diglucosyldiacylglycerol synthase in S. aureus providing glycolipid for LTA assembly. In LTA from the mutant, the glycolipid anchor is replaced by diacylglycerol. Although the doubling time of the mutant was identical to that of the wild type in Luria-Bertani (LB) medium, growth of the mutant in LB medium containing 1% glycine was not observed. This inhibition was antagonized by either L- or D-alanine. Moreover, viability of the mutant at 37 degrees C in 0.05 M phosphate (pH 7.2)-saline for 12 h was reduced to <0.1%. Addition of 0.1% D-glucose to the phosphate-saline ensured viability under these conditions. The autolysis of the ypfP::cat mutant in the presence of 0.05% Triton X-100 was 1.8-fold faster than that of the parental strain. Electron microscopy of the mutant revealed not only a small increase in cell size but also the presence of pleomorphic cells. Each of these phenotypes may be correlated with either (or both) a deficiency of free glycolipid in the membrane or the replacement of the usual glycolipid anchor of LTA with diacylglycerol.
Figures
FIG. 1
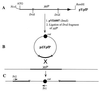
Cloning strategy for the expression and insertional inactivation of ypfP. (A) Fragment containing ypfP in pYpfP. The _Nco_I and _Bam_HI sites were introduced by PCR with the mutagenic primers described in Materials and Methods. (B) Cloning of the _Dra_I fragment of ypfP into the _Sma_I site of pVE6007 to obtain the plasmid for insertional inactivation (pΔYpfP). (C) Integration of pΔYpfP by a single crossover. Pr1 and Pr2 are primers used in the PCR analysis of integration. Pr1 is complementary to nt 1284 to 1265 of cat (chloramphenicol resistance gene) in pC194 (GenBank accession number J01754), and Pr2 is complementary to nt 2 to 21 of ypfP (GenBank accession number Y14370).
FIG. 2
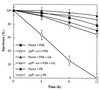
Viability of the ypfP::cat mutant in the presence of phosphate-saline buffer. The mutant (3 × 106 cells) and wild type (3 × 106 cells) were maintained in 0.05 M phosphate-saline buffer (pH 7.2) (PSB) at 37°C for the indicated time. The numbers of CFU were determined from LB agar plates and are reported as percent survivors (viability). For comparison, the wild type and mutant were also maintained in 0.05 M phosphate buffer (pH 7.2) (PB). In addition, 0.1%
d
-glucose (Glc) added to PSB containing the parent and mutant are compared.
FIG. 3
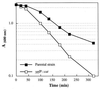
Triton X-100-stimulated cell autolysis of ypfP::cat and parental strains. Autolysis of whole cells was measured at 37°C in 50 mM Tris-HCl buffer (pH 7.2) containing 0.05% (vol/vol) Triton X-100 according to the method of Gustafson et al. (19).
FIG. 4
Scanning electron micrographs of exponential-phase cells of S. aureus RN4220 (A) and the ypfP::cat mutant (B). Bars, 1 μm. SEM was performed as described in Materials and Methods. Arrowheads in panel B show aberrant pleomorphs.
FIG. 5
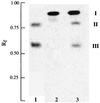
Incorporation of [14C]glucose from UDP-[14C]glucose into the glycolipids (A) and detection of α-naphthol-staining glycolipids (B). The synthesis of [14C]glucose-labeled glycolipids (Materials and Methods) was performed with 6 mg of permeabilized cells of either S. aureus (A, lane 1) or the ypfP::cat mutant (A, lane 2), with 20 μg of membranes from either the parent (A, lane 3) or the ypfP::cat mutant (A, lane 4), and with 20 μg of membranes from either E. coli containing pYpfP (A, lane 5) or E. coli BL21(DE3) (A, lane 6). The reaction conditions were 30 min at 37°C (lanes 1 to 4) and 5 min at 15°C for (lanes 5 and 6). I and III are MGlcDAG and DGlcDAG, respectively; II and IV were not identified. α-Naphthol-staining glycolipids from the parent (B, lane 1) and ypfP::cat mutant (B, lane 2) were compared with the standard β-gentiobiosyl diacylglycerol (DGlcDAG) (B, lane 3).
FIG. 6
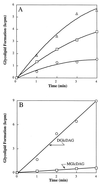
(A) Incorporation of [14C]glucose from UDP-[14C]glucose into MGlcDAG and DGlcDAG (summation) by recombinant YpfP; (B) incorporation of [14C]glucose from UDP-[14C]glucose into MGlcDAG and DGlcDAG by recombinant YpfP. In panel A, the reaction mixture (40 μl) contained 50 mM Tris-HCl buffer (pH 8.0), membrane-associated recombinant YpfP, and 0.35 nmol of UDP-[14C]glucose (286 mCi/mmol). Membranes concentrations were 2 (○), 4 (□), and 8 (▵) mg of protein/ml. The reaction mixtures were incubated at 15°C for 5 min. The radiolabeled glycolipids were extracted and analyzed by TLC as described in Materials and Methods.
FIG. 7
Incorporation of 1,2-di[1-14C]palmitoylglycerol into MGlcDAG and DGlcDAG by YpfP. The reaction mixture (40 μl) contained 1 mM UDP-glucose, 20 μg of membrane-associated recombinant YpfP, 3.7 pmol of 1,2 di[14C]palmitoylglycerol (937 cpm), and 44 mM Tris-HCl buffer (pH 8.0) containing 18% (vol/vol) glycerol. The mixtures were incubated at 37°C for 1 h. The incorporation of 1,2-di[1-14C]palmitoylglycerol (I) into II and III by the membranes from E. coli BL21(DE3) (lane 2) and from E. coli BL21(DE3) with YpfP (lane 3) is shown. The procedures for preparing the reaction mixture containing the radiolabeled diacylglycerol and the analyses of the lipids are described in Materials and Methods. For comparison, the incorporation of [14C]glucose from UDP-[14C]glucose into MGlcDAG (II) and DGlcDAG (III) by S. aureus membranes (lane 1) is shown.
FIG. 8
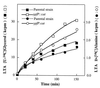
Release of
d
-[14C]alanine- and [14C]glycerol-labeled LTA by the ypfP::cat mutant and the parental strain. Samples of
d
-alanine (8,000 cpm)- and glycerol (36,000 cpm)-labeled cells were suspended in LB medium (2 ml) and grown with aeration at 37°C for the indicated times. Samples of the growth medium were separated from the cells using Nanosep MF centrifugal filters (0.45-μm pore size; Pall-Filtron), and the amounts of released radiolabel were quantified.
Similar articles
- A Staphylococcus aureus ypfP mutant with strongly reduced lipoteichoic acid (LTA) content: LTA governs bacterial surface properties and autolysin activity.
Fedtke I, Mader D, Kohler T, Moll H, Nicholson G, Biswas R, Henseler K, Götz F, Zähringer U, Peschel A. Fedtke I, et al. Mol Microbiol. 2007 Aug;65(4):1078-91. doi: 10.1111/j.1365-2958.2007.05854.x. Epub 2007 Jul 19. Mol Microbiol. 2007. PMID: 17640274 Free PMC article. - Genes required for glycolipid synthesis and lipoteichoic acid anchoring in Staphylococcus aureus.
Gründling A, Schneewind O. Gründling A, et al. J Bacteriol. 2007 Mar;189(6):2521-30. doi: 10.1128/JB.01683-06. Epub 2007 Jan 5. J Bacteriol. 2007. PMID: 17209021 Free PMC article. - Crucial Role for Lipoteichoic Acid Assembly in the Metabolic Versatility and Antibiotic Resistance of Staphylococcus aureus.
Burtchett TA, Shook JC, Hesse LE, Delekta PC, Brzozowski RS, Nouri A, Calas AJ, Spanoudis CM, Eswara PJ, Hammer ND. Burtchett TA, et al. Infect Immun. 2023 Jul 18;91(7):e0055022. doi: 10.1128/iai.00550-22. Epub 2023 Jun 22. Infect Immun. 2023. PMID: 37347167 Free PMC article. - Location, synthesis and function of glycolipids and polyglycerolphosphate lipoteichoic acid in Gram-positive bacteria of the phylum Firmicutes.
Reichmann NT, Gründling A. Reichmann NT, et al. FEMS Microbiol Lett. 2011 Jun;319(2):97-105. doi: 10.1111/j.1574-6968.2011.02260.x. Epub 2011 Mar 25. FEMS Microbiol Lett. 2011. PMID: 21388439 Free PMC article. Review. - The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus.
Xia G, Kohler T, Peschel A. Xia G, et al. Int J Med Microbiol. 2010 Feb;300(2-3):148-54. doi: 10.1016/j.ijmm.2009.10.001. Epub 2009 Nov 6. Int J Med Microbiol. 2010. PMID: 19896895 Review.
Cited by
- A Staphylococcus aureus ypfP mutant with strongly reduced lipoteichoic acid (LTA) content: LTA governs bacterial surface properties and autolysin activity.
Fedtke I, Mader D, Kohler T, Moll H, Nicholson G, Biswas R, Henseler K, Götz F, Zähringer U, Peschel A. Fedtke I, et al. Mol Microbiol. 2007 Aug;65(4):1078-91. doi: 10.1111/j.1365-2958.2007.05854.x. Epub 2007 Jul 19. Mol Microbiol. 2007. PMID: 17640274 Free PMC article. - Bacillus subtilis alpha-phosphoglucomutase is required for normal cell morphology and biofilm formation.
Lazarevic V, Soldo B, Médico N, Pooley H, Bron S, Karamata D. Lazarevic V, et al. Appl Environ Microbiol. 2005 Jan;71(1):39-45. doi: 10.1128/AEM.71.1.39-45.2005. Appl Environ Microbiol. 2005. PMID: 15640167 Free PMC article. - Enzymatic activities and functional interdependencies of Bacillus subtilis lipoteichoic acid synthesis enzymes.
Wörmann ME, Corrigan RM, Simpson PJ, Matthews SJ, Gründling A. Wörmann ME, et al. Mol Microbiol. 2011 Feb;79(3):566-83. doi: 10.1111/j.1365-2958.2010.07472.x. Epub 2010 Dec 7. Mol Microbiol. 2011. PMID: 21255105 Free PMC article. - Pleiotropic roles of polyglycerolphosphate synthase of lipoteichoic acid in growth of Staphylococcus aureus cells.
Oku Y, Kurokawa K, Matsuo M, Yamada S, Lee BL, Sekimizu K. Oku Y, et al. J Bacteriol. 2009 Jan;191(1):141-51. doi: 10.1128/JB.01221-08. Epub 2008 Oct 24. J Bacteriol. 2009. PMID: 18952789 Free PMC article. - GtcA is required for LTA glycosylation in Listeria monocytogenes serovar 1/2a and Bacillus subtilis.
Rismondo J, Haddad TFM, Shen Y, Loessner MJ, Gründling A. Rismondo J, et al. Cell Surf. 2020 Feb 19;6:100038. doi: 10.1016/j.tcsw.2020.100038. eCollection 2020 Dec. Cell Surf. 2020. PMID: 32743150 Free PMC article.
References
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
- Archibald A R, Baddiley J, Heptinstall S. The alanine ester content and magnesium binding capacity of walls of Staphylococcus aureus H grown at different pH values. Biochim Biophys Acta. 1973;291:629–634. - PubMed
- Baird-Parker A C. An improved diagnostic and selective medium for isolating coagulase positive staphylococci. J Appl Bacteriol. 1962;25:12–19.
- Bierbaum G, Sahl H-G. Induction of autolysis of Staphylococcus simulans 22 by Pep5 and nisin and influence of the cationic peptides on the activity of the autolytic enzymes, p 386–396. In: Jung G, Sahl H-G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM; 1991.
- Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous