Protein kinase PKR is required for platelet-derived growth factor signaling of c-fos gene expression via Erks and Stat3 - PubMed (original) (raw)
Protein kinase PKR is required for platelet-derived growth factor signaling of c-fos gene expression via Erks and Stat3
A Deb et al. EMBO J. 2001.
Abstract
The double-stranded RNA (dsRNA)-activated protein kinase PKR is an interferon (IFN)-induced enzyme that controls protein synthesis through phosphorylation of eukaryotic initiation factor 2alpha (eIF-2alpha). PKR also regulates signals initiated by diverse stimuli, including dsRNA, IFN-gamma, tumor necrosis factor-alpha, interleukin-1 and lipopolysaccharide, to different transcription factors, resulting in pro-inflammatory gene expression. Stat3 plays an essential role in promoting cell survival and proliferation by different growth factors, including platelet-derived growth factor (PDGF). Here we show that PKR physically interacts with Stat3 and is required for PDGF-induced phosphorylation of Stat3 at Tyr705 and Ser727, resulting in DNA binding and transcriptional activation. PKR-mediated phosphorylation of Stat3 on Ser727 is indirect and channeled through ERKS: Although PKR is pre-associated with the PDGF beta-receptor, treatment with PDGF only modestly activates PKR. However, the induction of c-fos by PDGF is defective in PKR-null cells. Taken together, these results establish PKR as an upstream regulator of activation of Stat3 and as a common mediator of both growth-promoting and growth-inhibitory signals.
Figures
Fig. 1. PKR is required for Erk activation and Ser727 and Tyr705 phosphorylation of Stat3 in response to PDGF. (A) PKR+/+ and PKRo/o MEFs were treated with PDGF for the indicated times, whole-cell extracts (100 µg) were separated by SDS–PAGE, and phosphorylation of Stat3 at Tyr705 was assayed by immunoblotting. The two forms of Stat3 (Stat3fm and Stat3sm) recognized by this antibody in PKR+/+ MEFs are indicated. The blot was re-probed with anti Stat3 for normalization. (B) Extracts of PDGF-treated PKR+/+ and PKRo/o MEFs were immunoblotted with antibody specific for pSer727 Stat3 (top panel), anti-Stat3 (lower panel). (C) PKR+/+ MEFs were pre-treated with 10 µM SB 203580 (p38 inhibitor) and 30 µM PD 098059 (Erk inhibitor) for 30 min before treatment with PDGF for 15 min. Extracts were separated by SDS–PAGE, transferred onto nylon membranes and immunoblotted with anti pSer727 Stat3 (top panel), anti-Stat3 (middle panel) and anti-phospho-Erk1/2 antibodies (bottom panel). (D) Extracts of PDGF-treated PKR+/+ and PKRo/o MEFs were immunoblotted with antibody specific for phosphorylated (activated) forms of Erk1/2.
Fig. 2. PKR and Stat3 physically associate. (A) Whole-cell lysates (500 µg) from Daudi cells were used for immunoprecipitation with an anti-Stat3 monoclonal antibody (mAb), anti-Stat3 polyclonal antibody (pAb) and anti-PKR mAb. Immunoprecipitates were separated by SDS–PAGE and transferred to nylon membranes. The lower form of Stat3, Stat3β, is not recognized in the immunoprecipitation with anti-Stat3 pAb as it is raised against a C-terminal region of Stat3 that is missing in Stat3β. (B) Extracts from different human cell lines immunoprecipitated with anti-Stat3 antibody (mAb) and separated by SDS–PAGE, transferred to nylon membranes and immunoblotted with anti-PKR (pAb, upper panel) and anti-Stat3 (mAb, lower panel). (C) Whole-cell extracts from PKR MEFs or cell lines derived from MEFs were immunoprecipitated with anti-Stat3 antibody (mAb) followed by immunoblotting with anti-PKR (pAb, mouse, upper panel) or anti-Stat3 (mAb, lower panel). A control lane showing immuno precipitated PKR is also shown (last lane) (D) Whole-cell lysates from PKRo/o cells stably expressing human PKR from its natural promoter (Bac9) were immunoprecipitated with anti-PKR (mAb) antibody followed by immunoblotting with anti-PKR (pAb, upper panel) and anti-Stat3 (mAb, lower panel) antibodies. Bac10 cells do not express any PKR and served as control. (E) Serum-starved MEFs were treated with PDGF for different times, whole-cell lysates were immunoprecipitated with anti-Stat3 antibody (mAb), and immunoprecipitates were analyzed by immunoblotting with anti-mouse PKR (pAb, upper panel) or anti-Stat3 (mAb, lower panel) antibodies.
Fig. 3. PKR associates with the PDGF β-receptor in a constitutive manner. (A) Bac9 and Bac10 cells were serum starved overnight and then treated with PDGF (20 ng/ml) as described in Materials and methods. Whole-cell extracts (1 mg, left side; 400 µg, right side) were immunoprecipitated with anti-PDGFR β antibody (pAb). Immunoprecipitates were immunoblotted with PDGFR antibody (top panel, left side) or PKR antibody (BCI, lower panel, left side). The figure on the right shows that the receptor was tyrosine phosphorylated in response to PDGF. The membrane was first probed for phosphotyrosines (PY20 and 4G10 polyclonal) before being stripped and re-probed for total PDGFR β protein. NS refers to a non-specific cross-reacting protein band. (B) PKR+/+ and PKRo/o MEFs were serum starved for 18 h and treated with PDGF (20 ng/ml) for the indicated times. Whole-cell extracts (300 µg) were immunoprecipitated with anti-PDGFR β antibody and subjected to SDS–PAGE analysis. The top portion of the blot was probed initially for tyrosine-phosphorylated receptor (top panel) and then stripped and re-probed for total receptor (middle panel). The lower half of the blot was immunoprobed for PKR using a mouse polyclonal antibody raised against mouse PKR (lower panel). (C) Mouse PKR-null cells reconstituted for PKR by stable transfection of a BAC clone carrying human PKR gene (Bac9) were treated with PDGF (20 ng/ml) or pIC (100 µg/ml) following overnight starvation. In vitro kinase assays were performed on immunoprecipitated PKR (from 150 µg of extract) as described in Materials and methods. Following autoradiography (top panel), the membrane was immunoprobed for the presence of PKR protein in each IP (lower panel). The graph reflects the mean phosphorimager quantitation of autophosphorylated PKR from three separate experiments, each normalized for the amount of immunoprecipitated PKR.
Fig. 4. PKR is required for induction of c-fos but not mitogenesis in response to PDGF. (A) Total RNA was isolated from PDGF-treated PKR+/+ and PKRo/o cells and used for RNase protection assays as described in Materials and methods. Products were analyzed on 8% polyacrylamide–8 M urea gels. (B) Quantitation of normalized values for c-fos induction from phosphorimager analysis. (C and D) PKR+/+ and PKRo/o MEFs were grown in 0.3% FBS-containing medium to∼50–60% confluency before the addition of serum (10%) or PDGF (20 ng/ ml) and further incubation for 24 h in the presence of BrdU. Cells were fixed and processed for immunofluorescence as described in Materials and methods. An average of 10 fields was taken to count the percentage of cells that had incorporated BrdU. BrdU labeling index (%) is the ratio of the number of BrdU-labeled cells in a given field and the total number of cells in the same field.
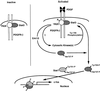
Fig. 5. Schematic representation of the role of PKR in PDGF signaling. PKR and Stat3 are constitutively associated with the PDGF β-receptor. PKR is required for both serine and tyrosine phosphorylation of Stat3 in response to PDGF. The interaction of PKR and Stat3 on the PDGF β-receptor may be a part of a large complex involving additional factors not shown in the model. The open, hatched and striped molecules represent PKR, Stat3 and Stat1 proteins, respectively.
Similar articles
- Platelet-derived growth factor signal transduction through the interferon-inducible kinase PKR. Immediate early gene induction.
Mundschau LJ, Faller DV. Mundschau LJ, et al. J Biol Chem. 1995 Feb 17;270(7):3100-6. doi: 10.1074/jbc.270.7.3100. J Biol Chem. 1995. PMID: 7531699 - Activation of Stat3 preassembled with platelet-derived growth factor beta receptors requires Src kinase activity.
Wang YZ, Wharton W, Garcia R, Kraker A, Jove R, Pledger WJ. Wang YZ, et al. Oncogene. 2000 Apr 20;19(17):2075-85. doi: 10.1038/sj.onc.1203548. Oncogene. 2000. PMID: 10815799 - STAT activation by the PDGF receptor requires juxtamembrane phosphorylation sites but not Src tyrosine kinase activation.
Sachsenmaier C, Sadowski HB, Cooper JA. Sachsenmaier C, et al. Oncogene. 1999 Jun 17;18(24):3583-92. doi: 10.1038/sj.onc.1202694. Oncogene. 1999. PMID: 10380880 - PKR; a sentinel kinase for cellular stress.
Williams BR. Williams BR. Oncogene. 1999 Nov 1;18(45):6112-20. doi: 10.1038/sj.onc.1203127. Oncogene. 1999. PMID: 10557102 Review. - Roles of protein kinase R in cancer: Potential as a therapeutic target.
Watanabe T, Imamura T, Hiasa Y. Watanabe T, et al. Cancer Sci. 2018 Apr;109(4):919-925. doi: 10.1111/cas.13551. Epub 2018 Mar 23. Cancer Sci. 2018. PMID: 29478262 Free PMC article. Review.
Cited by
- PKR activation-induced mitochondrial dysfunction in HIV-transgenic mice with nephropathy.
Yoshida T, Latt KZ, Rosenberg AZ, Santo BA, Myakala K, Ishimoto Y, Zhao Y, Shrivastav S, Jones BA, Yang X, Wang XX, Tutino VM, Sarder P, Levi M, Okamoto K, Winkler CA, Kopp JB. Yoshida T, et al. Elife. 2024 Aug 29;12:RP91260. doi: 10.7554/eLife.91260. Elife. 2024. PMID: 39207915 Free PMC article. - Time-resolved Phosphoproteome Analysis of Paradoxical RAF Activation Reveals Novel Targets of ERK.
Kubiniok P, Lavoie H, Therrien M, Thibault P. Kubiniok P, et al. Mol Cell Proteomics. 2017 Apr;16(4):663-679. doi: 10.1074/mcp.M116.065128. Epub 2017 Feb 10. Mol Cell Proteomics. 2017. PMID: 28188228 Free PMC article. - The Potential Role of Protein Kinase R as a Regulator of Age-Related Neurodegeneration.
Martinez NW, Gómez FE, Matus S. Martinez NW, et al. Front Aging Neurosci. 2021 Apr 28;13:638208. doi: 10.3389/fnagi.2021.638208. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33994991 Free PMC article. Review. - Effect of sinomenine on vascular smooth muscle cell dedifferentiation and neointima formation after vascular injury in mice.
Zhu L, Hao Y, Guan H, Cui C, Tian S, Yang D, Wang X, Zhang S, Wang L, Jiang H. Zhu L, et al. Mol Cell Biochem. 2013 Jan;373(1-2):53-62. doi: 10.1007/s11010-012-1474-9. Epub 2012 Oct 13. Mol Cell Biochem. 2013. PMID: 23065380 - Dance with the Devil: Stress Granules and Signaling in Antiviral Responses.
Eiermann N, Haneke K, Sun Z, Stoecklin G, Ruggieri A. Eiermann N, et al. Viruses. 2020 Sep 4;12(9):984. doi: 10.3390/v12090984. Viruses. 2020. PMID: 32899736 Free PMC article. Review.
References
- Bandyopadhyay S.K., de La Motte,C.A. and Williams,B.R. (2000) Induction of E-selectin expression by double stranded RNA and TNF-α is attenuated in murine aortic endothelial cells derived from protein kinase PKR-null mice. J. Immunol., 164, 2077–2083. - PubMed
- Bowman T., Garcia,R., Turkson,J. and Jove,R. (2000) STATs in oncogenesis. Oncogene, 19, 2474–2488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous