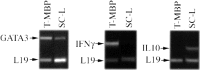Protective autoimmunity is a physiological response to CNS trauma - PubMed (original) (raw)
Protective autoimmunity is a physiological response to CNS trauma
E Yoles et al. J Neurosci. 2001.
Erratum in
- J Neurosci 2001 Aug 1;21(15):1a
Abstract
Primary damage caused by injury to the CNS is often followed by delayed degeneration of initially spared neurons. Studies in our laboratory have shown that active or passive immunization with CNS myelin-associated self-antigens can reduce this secondary loss. Here we show, using four experimental paradigms in rodents, that CNS trauma spontaneously evokes a beneficial T cell-dependent immune response, which reduces neuronal loss. (1) Survival of retinal ganglion cells in rats was significantly higher when optic nerve injury was preceded by an unrelated CNS (spinal cord) injury. (2) Locomotor activity of rat hindlimbs (measured in an open field using a locomotor rating scale) after contusive injury of the spinal cord (T8) was significantly better (by three to four score grades) after passive transfer of myelin basic protein (MBP)-activated splenocytes derived from spinally injured rats than in untreated injured control rats or rats similarly treated with splenocytes from naive animals or with splenocytes from spinally injured rats activated ex vivo with ovalbumin or without any ex vivo activation. (3) Neuronal survival after optic nerve injury was 40% lower in adult rats devoid of mature T cells (caused by thymectomy at birth) than in normal rats. (4) Retinal ganglion cell survival after optic nerve injury was higher (119 +/- 3.7%) in transgenic mice overexpressing a T cell receptor (TcR) for MBP and lower (85 +/- 1.3%) in mice overexpressing a T cell receptor for the non-self antigen ovalbumin than in matched wild types. Taken together, the results imply that CNS injury evokes a T cell-dependent neuroprotective response.
Figures
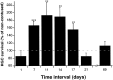
Fig. 1.
Systemic neuroprotection evoked by CNS injury in male rats. Male SPD rats were subjected to unilateral partial crush injury of the optic nerve 1−69 d after spinal cord contusion. Two weeks after the crush injury the number of surviving neurons was determined by retrograde labeling of their RGCs. The numbers of rats used at each time point (contused/control) were as follows: day 1, 14/17; day 7, 11/10; day 11, 10/11; day 14, 10/10; day 17, 14/15; day 21, 8/9; day 69, 9/12. At each time point, the ratio between the mean number of RGCs in the retinas of contused rats (i.e., where the optic nerve lesion was preceded by a spinal cord contusion) and the mean number of RGCs in control rats (where the optic nerve lesion was preceded at the corresponding time point by anesthesia only) was calculated. The results are expressed as mean percentages ± SE. The mean numbers of viable fibers (reflected by labeled RGCs) in all the control groups were similar, regardless of the interval between anesthesia and optic nerve crush (50.6, 57.3, 42.53, 58.17, 45.52, 43.25; mean ± SEM for all experiments, 48.85 ± 3.05). The time lapse between the two injuries significantly affected neuronal survival after the optic nerve injury (p < 0.008; ANOVA). Comparison between the groups revealed significantly better neuronal survival when the time lapse between the two injuries was 7–17 d.
Fig. 2.
The immune neuroprotection evoked by spinal cord contusion is transferable in SPD rats. A, Rats were subjected to spinal cord contusion. One week later their spleens were excised and incubated in vitro with PECs as antigen-presenting cells and with MBP. After 3 d the activated spleen cells were collected, washed, and counted. The resuspended cell population (4.5 × 107) or PBS was injected into rats newly subjected to spinal contusion (n = 5 for each group). In a separate experiment (B), similarly prepared splenocytes from spinally contused rats activated ex vivo with MBP (Contused-MBP; n = 4) or OVA (Contused-OVA; n = 5), or splenocytes prepared from sham-operated animals activated with MBP (Naive-MBP; n = 5), were transferred to newly contused rats. A group of newly contused rats injected with PBS (n = 5) was also included. No effect was seen with splenocytes from contused rats incubated in vitro_with OVA. Similarly prepared splenocytes, withdrawn from rats 14 d (C) or 3 d (D) after spinal contusion, were activated with irradiated thymocytes in the presence of MBP. After 3 d in vitro the activated spleen cells were collected, washed, and counted. The resuspended cells were injected into six rats newly subjected to spinal contusion (4.5 × 107 cells per rat). In each experiment (C, D), five rats that were subjected to spinal contusion and injected with the vehicle (PBS) served as control. E, Transfer of splenocytes withdrawn 14 d after spinal cord contusion and injected with no previous_ex vivo activation into five rats newly subjected to spinal cord contusion. Five rats that were subjected to spinal contusion and injected with the vehicle (PBS) served as control. Behavioral outcome was evaluated according to a double-blind protocol, using a locomotor test with scores ranging from 0 to 21 (see Materials and Methods). Results are expressed as the mean ± SE at each time point tested. Repeated-measures ANOVA revealed significant effect (p < 0.05) in A–C and no effect in_D_ and E.
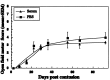
Fig. 3.
Passive transfer of antibodies does not confer neuroprotection. Lewis rats were immunized with MBP in incomplete Freund's adjuvant. After 60 d, 2 ml of sera pooled from seven rats was injected intravenously into rats newly subjected to spinal cord contusion. Six control rats were injected with PBS. At the indicated time periods, rats were evaluated by a locomotor test with scores ranging from 0 to 21 (see Materials and Methods). Behavioral outcome was assessed by observers blinded to the treatment received by the rats. Results are expressed as the mean ± SE at each time point tested (n = 6 for each group).
Fig. 4.
Expression of GATA-3 and IL-10, but not of IFNγ, by lymphocytes isolated from contused spinal cords. Lymphocytes were isolated from spinal cords 7 d after spinal cord contusion (SC-L). RNA extracted from the isolated cells was subjected to RT−PCR. RNA extracted from a T cell line directed to MBP [known to express both pro-inflammatory and anti-inflammatory cytokines (Moalem et al., 2000b)] was used as control. The lymphocytes recovered from contused spinal cords expressed mostly IL-10 and hardly any IFNγ.
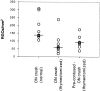
Fig. 5.
Adult rats subjected to thymectomy at birth recover poorly from CNS injury. Thymectomized rats (n = 18) were subjected to a partial crush injury of their optic nerves. One week before the optic nerve crush, 8 of these rats underwent spinal cord contusion (precontused ON crush; thymectomized) and 10 underwent a sham operation (ON crush; thymectomized). Two weeks after optic nerve injury the surviving neurons were labeled, and 5 d later the retinas were excised and their RGCs counted. Normal rats (n = 9) were subjected to optic nerve crush only (ON crush). The number of RGCs per square millimeter in each animal is shown. Thymectomy had a significant effect on RGC survival, both in the absence of previous contusion (p < 0.003) and in the precontused group (p < 0.01; t test).
Fig. 6.
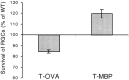
Survival of retinal ganglion cells after optic nerve crush in transgenic mice overexpressing a receptor for anti-MBP T cells or anti-OVA T cells. All RGCs of both eyes of transgenic mice overexpressing a T cell receptor for MBP or a T cell receptor for OVA and of the corresponding wild-type mice were labeled with a stereotactically injected dye. Three days later the mice were subjected to a unilateral crush injury. After 1 week the retinas were excised and whole mounted, and their labeled (surviving) RGCs were counted. The mean numbers of RGCs ± SEM in each group of transgenic mice are expressed here as percentages of the matched wild type. Survival of the injured mice overexpressing a T cell receptor for MBP (n = 10) was significantly higher than in the corresponding wild type (n = 10;p < 0.009), whereas in mice overexpressing a T cell receptor for OVA (n = 9) survival was lower than in the corresponding wild type (n = 10;p < 0.0003; t test).
Similar articles
- Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response.
Kipnis J, Yoles E, Schori H, Hauben E, Shaked I, Schwartz M. Kipnis J, et al. J Neurosci. 2001 Jul 1;21(13):4564-71. doi: 10.1523/JNEUROSCI.21-13-04564.2001. J Neurosci. 2001. PMID: 11425884 Free PMC article. - Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system.
Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Kipnis J, et al. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15620-5. doi: 10.1073/pnas.232565399. Epub 2002 Nov 12. Proc Natl Acad Sci U S A. 2002. PMID: 12429857 Free PMC article. - Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion.
Hauben E, Butovsky O, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Leibowitz-Amit R, Pevsner E, Akselrod S, Neeman M, Cohen IR, Schwartz M. Hauben E, et al. J Neurosci. 2000 Sep 1;20(17):6421-30. doi: 10.1523/JNEUROSCI.20-17-06421.2000. J Neurosci. 2000. PMID: 10964948 Free PMC article. - T cell-based therapeutic vaccination for spinal cord injury.
Schwartz M, Hauben E. Schwartz M, et al. Prog Brain Res. 2002;137:401-6. doi: 10.1016/s0079-6123(02)37031-6. Prog Brain Res. 2002. PMID: 12440382 Review. - Autoimmunity can benefit self-maintenance.
Schwartz M, Cohen IR. Schwartz M, et al. Immunol Today. 2000 Jun;21(6):265-8. doi: 10.1016/s0167-5699(00)01633-9. Immunol Today. 2000. PMID: 10825737 Review.
Cited by
- Modulation of neural stem/progenitor cell proliferation during experimental Herpes Simplex encephalitis is mediated by differential FGF-2 expression in the adult brain.
Rotschafer JH, Hu S, Little M, Erickson M, Low WC, Cheeran MC. Rotschafer JH, et al. Neurobiol Dis. 2013 Oct;58:144-55. doi: 10.1016/j.nbd.2013.05.018. Epub 2013 Jun 5. Neurobiol Dis. 2013. PMID: 23748078 Free PMC article. - Release of interleukin-10 and neurotrophic factors in the choroid plexus: possible inductors of neurogenesis following copolymer-1 immunization after cerebral ischemia.
Cruz Y, García EE, Gálvez JV, Arias-Santiago SV, Carvajal HG, Silva-García R, Bonilla-Jaime H, Rojas-Castañeda J, Ibarra A. Cruz Y, et al. Neural Regen Res. 2018 Oct;13(10):1743-1752. doi: 10.4103/1673-5374.238615. Neural Regen Res. 2018. PMID: 30136689 Free PMC article. - Administration of anti-ERMAP antibody ameliorates Alzheimer's disease in mice.
Liu H, Zhao J, Lin Y, Su M, Lai L. Liu H, et al. J Neuroinflammation. 2021 Nov 13;18(1):268. doi: 10.1186/s12974-021-02320-x. J Neuroinflammation. 2021. PMID: 34774090 Free PMC article. - Immune Memory in Aging: a Wide Perspective Covering Microbiota, Brain, Metabolism, and Epigenetics.
Bulut O, Kilic G, Domínguez-Andrés J. Bulut O, et al. Clin Rev Allergy Immunol. 2022 Dec;63(3):499-529. doi: 10.1007/s12016-021-08905-x. Epub 2021 Dec 15. Clin Rev Allergy Immunol. 2022. PMID: 34910283 Free PMC article. Review. - T cell memory in the injured facial motor nucleus: relation to functional recovery following facial nerve crush.
Ha GK, Pastrana M, Huang Z, Petitto JM. Ha GK, et al. Neurosci Lett. 2008 Oct 10;443(3):150-4. doi: 10.1016/j.neulet.2008.07.067. Epub 2008 Jul 31. Neurosci Lett. 2008. PMID: 18687384 Free PMC article.
References
- Aguayo AJ, Rasminsky M, Bray GM, Carbonetto S, McKerracher L, Villegas-Perez MP, Vidal-Sanz M, Carter DA. Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Philos Trans R Soc Lond B Biol Sci. 1991;331:337–343. - PubMed
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. - PubMed
- Beattie MS, Bresnahan JC, Komon J, Tovar CA, Van Meter M, Anderson DK, Faden AI, Hsu CY, Noble LJ, Salzman S, Young S. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol. 1997;148:453–463. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous