Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levels in the visual system, hippocampus, and cerebellum - PubMed (original) (raw)
Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levels in the visual system, hippocampus, and cerebellum
G S Pollock et al. J Neurosci. 2001.
Abstract
The expression of brain-derived neurotrophic factor (BDNF) mRNA and the secretion of BDNF protein are tightly regulated by neuronal activity. Thus, BDNF has been proposed as a mediator of activity-dependent neural plasticity. Previous studies showed that dark rearing (DR) reduces BDNF mRNA levels in the primary visual cortex (V1), but the effects of visual experience on BDNF protein levels are unknown. We report that rearing in constant light or DR alters BDNF mRNA and protein levels in the retina, superior colliculus (SC), V1, hippocampus (HIPP), and cerebellum (CBL), although the changes in mRNA and protein are not always correlated. Most notably, DR increases BDNF protein levels in V1 although BDNF mRNA is decreased. BDNF protein levels also undergo diurnal changes. In the retina, V1, and SC, BDNF protein levels are higher during the light phase of the circadian cycle than during the dark phase. By contrast, in HIPP and CBL, the tissue concentration of BDNF protein is higher during the dark phase. The discrepancies between the experience-dependent changes in BDNF mRNA and protein suggest that via its effects on neuronal activity, early sensory experience alters the trafficking, as well as the synthesis, of BDNF protein. The circadian changes in BDNF protein suggest that BDNF could cause the diurnal modulation of synaptic efficacy in some neural circuits. The fluctuations in BDNF levels in nonvisual structures suggest a potential role of BDNF in mediating plasticity induced by hormones or motor activity.
Figures
Fig. 1.
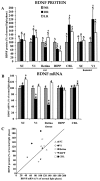
A, ECLIA measurements of BDNF protein concentrations in the SC, V1, retina, HIPP, and CBL of rats born and reared in a normal light cycle (NR; 14/10 hr light/dark cycle), constant darkness (DR), or constant light (LR). Similar measurements for SC and V1 are presented for hamsters at the right. All values are expressed as the percentage of normal. Error bars show SD. _Numbers above each bar_indicate the number of independent samples from which data were obtained (see Materials and Methods). B, RPA measurements of BDNF mRNA concentrations in rats in the same structures and under the same rearing conditions shown in A. All values are expressed as the percentage of normal. Numbers above each bar indicate the number of independent samples from which data were obtained (see Materials and Methods). Means and SEs of PhosphorImager pixel densities are indicated in Table 1.C, Scatter plot summarizing the relationship between changes in BDNF mRNA and BDNF protein induced in rats by DR (filled symbols) and LR (open symbols). Asterisks in A and _B_indicate values in LR or DR animals that differ significantly from normal.
Fig. 2.
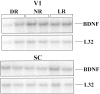
Protected mRNA fragments corresponding to BDNF and the L32 housekeeping protein, obtained by performing the ribonuclease protection assay on mRNA from rat V1 (top) and rat SC (bottom). Two cases are shown for each tissue for each of the three rearing conditions (NR, DR, and LR). Gels were aligned using labeled standards (data not shown). Densitometric data were corrected as described in Materials and Methods for variations in mRNA loading, as measured by the L32 signal.
Fig. 3.
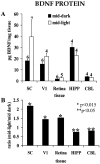
Circadian modulation of BDNF protein levels.A, Tissue concentrations of BDNF protein in hamster SC, V1, retina, HIPP, and CBL in the middle of the light and dark phases of the diurnal cycle. Numerals above each bar indicate the number of independent samples from which data were obtained (see Materials and Methods). B, Ratios of the mean BDNF protein concentration in the middle of the light phase of the diurnal cycle to the concentration in the middle of the dark phase.Asterisks indicate the level of statistical significance for differences between light- and dark-phase measurements in_A_ (*p < 0.015; **p < 0.05).
Fig. 4.
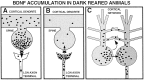
Mechanisms that might account for the accumulation of BDNF protein in V1 of dark-reared animals. A, A reduction in the removal of BDNF by LGN or other afferents (arrow across the synapse). Only axodendritic synapses are depicted, but axosomatic connections are also present.B, An accumulation of BDNF delivered to LGN or other afferent presynaptic terminals by anterograde axonal transport.C, An increase in the retention of locally synthesized BDNF within cortical networks by transsynaptic, autocrine, or paracrine secretion (arrows between cells). Increased cortical BDNF could also arise by reduced anterograde transport or increased retrograde transport in the axons of cortical efferent neurons (arrow in axon).
Similar articles
- Complexity in the modulation of neurotrophic factor mRNA expression by early visual experience.
Pollock GS, Frost DO. Pollock GS, et al. Brain Res Dev Brain Res. 2003 Jul 12;143(2):225-32. doi: 10.1016/s0165-3806(03)00153-6. Brain Res Dev Brain Res. 2003. PMID: 12855194 - A phase advance of the light-dark cycle stimulates production of BDNF, but not of other neurotrophins, in the adult rat cerebral cortex: association with the activation of CREB.
Katoh-Semba R, Tsuzuki M, Miyazaki N, Matsuda M, Nakagawa C, Ichisaka S, Sudo K, Kitajima S, Hamatake M, Hata Y, Nagata K. Katoh-Semba R, et al. J Neurochem. 2008 Sep;106(5):2131-42. doi: 10.1111/j.1471-4159.2008.05565.x. Epub 2008 Jul 12. J Neurochem. 2008. PMID: 18636983 - Mismatch between BDNF mRNA and protein expression in the developing visual cortex: the role of visual experience.
Tropea D, Capsoni S, Tongiorgi E, Giannotta S, Cattaneo A, Domenici L. Tropea D, et al. Eur J Neurosci. 2001 Feb;13(4):709-21. doi: 10.1046/j.0953-816x.2000.01436.x. Eur J Neurosci. 2001. PMID: 11207806 - Rapid regulation of brain-derived neurotrophic factor mRNA within eye-specific circuits during ocular dominance column formation.
Lein ES, Shatz CJ. Lein ES, et al. J Neurosci. 2000 Feb 15;20(4):1470-83. doi: 10.1523/JNEUROSCI.20-04-01470.2000. J Neurosci. 2000. PMID: 10662837 Free PMC article. - Pleiotropic effects of BDNF on the cerebellum and hippocampus: Implications for neurodevelopmental disorders.
Camuso S, La Rosa P, Fiorenza MT, Canterini S. Camuso S, et al. Neurobiol Dis. 2022 Feb;163:105606. doi: 10.1016/j.nbd.2021.105606. Epub 2021 Dec 30. Neurobiol Dis. 2022. PMID: 34974125 Review.
Cited by
- Targeting the circadian modulation: novel therapeutic approaches in the management of ASD.
Zhang Y, Chen Y, Li W, Tang L, Li J, Feng X. Zhang Y, et al. Front Psychiatry. 2024 Oct 11;15:1451242. doi: 10.3389/fpsyt.2024.1451242. eCollection 2024. Front Psychiatry. 2024. PMID: 39465045 Free PMC article. Review. - Circadian rhythm of brain-derived neurotrophic factor in serum and plasma.
Ehrhardt M, Schreiber S, Duderstadt Y, Braun-Dullaeus R, Borucki K, Brigadski T, Müller NG, Leßmann V, Müller P. Ehrhardt M, et al. Exp Physiol. 2024 Oct;109(10):1755-1767. doi: 10.1113/EP091671. Epub 2024 Aug 6. Exp Physiol. 2024. PMID: 39105714 Free PMC article. - Composite Coatings Based on Recombinant Spidroins and Peptides with Motifs of the Extracellular Matrix Proteins Enhance Neuronal Differentiation of Neural Precursor Cells Derived from Human Induced Pluripotent Stem Cells.
Novosadova EV, Dolotov OV, Novosadova LV, Davydova LI, Sidoruk KV, Arsenyeva EL, Shimchenko DM, Debabov VG, Bogush VG, Tarantul VZ. Novosadova EV, et al. Int J Mol Sci. 2023 Mar 2;24(5):4871. doi: 10.3390/ijms24054871. Int J Mol Sci. 2023. PMID: 36902300 Free PMC article. - High-Contrast Stimulation Potentiates the Neurotrophic Properties of Müller Cells and Suppresses Their Pro-Inflammatory Phenotype.
Zloh M, Kutilek P, Stofkova A. Zloh M, et al. Int J Mol Sci. 2022 Aug 3;23(15):8615. doi: 10.3390/ijms23158615. Int J Mol Sci. 2022. PMID: 35955747 Free PMC article. - Brain-Derived Neurotrophic Factor-Mediated Neuroprotection in Glaucoma: A Review of Current State of the Art.
Lambuk L, Mohd Lazaldin MA, Ahmad S, Iezhitsa I, Agarwal R, Uskoković V, Mohamud R. Lambuk L, et al. Front Pharmacol. 2022 May 20;13:875662. doi: 10.3389/fphar.2022.875662. eCollection 2022. Front Pharmacol. 2022. PMID: 35668928 Free PMC article. Review.
References
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. - PubMed
- Androutsellis-Theotokis A, McCormack WJ, Bradford HF, Stern GM, Pliego-Rivero F. The depolarisation-induced release of [125I]BDNF from brain tissue. Brain Res. 1996;743:40–48. - PubMed
- Bear MF, Cooper LN, Ebner FF. A physiological basis for a theory of synapse modification. Science. 1987;237:42–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS07375/NS/NINDS NIH HHS/United States
- EY11434/EY/NEI NIH HHS/United States
- MH49568/MH/NIMH NIH HHS/United States
- T32 NS007375/NS/NINDS NIH HHS/United States
- EY11127/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous