Influenza A virus can undergo multiple cycles of replication without M2 ion channel activity - PubMed (original) (raw)
Influenza A virus can undergo multiple cycles of replication without M2 ion channel activity
T Watanabe et al. J Virol. 2001 Jun.
Abstract
Ion channel proteins are common constituents of cells and have even been identified in some viruses. For example, the M2 protein of influenza A virus has proton ion channel activity that is thought to play an important role in viral replication. Because direct support for this function is lacking, we attempted to generate viruses with defective M2 ion channel activity. Unexpectedly, mutants with apparent loss of M2 ion channel activity by an in vitro assay replicated as efficiently as the wild-type virus in cell culture. We also generated a chimeric mutant containing an M2 protein whose transmembrane domain was replaced with that from the hemagglutinin glycoprotein. This virus replicated reasonably well in cell culture but showed no growth in mice. Finally, a mutant lacking both the transmembrane and cytoplasmic domains of M2 protein grew poorly in cell culture and showed no growth in mice. Thus, influenza A virus can undergo multiple cycles of replication without the M2 transmembrane domain responsible for ion channel activity, although this activity promotes efficient viral replication.
Figures
FIG. 1
Schematic diagram of mutant influenza virus M2 proteins and their properties. The amino acid sequence of the TM domain (residues 25 to 43) is shown in single-letter code in the expanded section of the diagram. Ion channel activity was determined by Holsinger et al. (18) using a two-electrode voltage clamp procedure. +, detectable ion channel activity; −, no detectable ion channel activity.
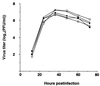
FIG. 2
Growth curves of M2 mutant and wild-type WSN-UdM viruses. MDCK cells were infected with virus at an MOI of 0.001. At the indicated times after infection, the virus titer in the supernatant was determined. The values are means of triplicate experiments. The standard deviation (SD) is less than 0.59 for each sample. □, M2V27T; ■, M2A30P; +, M2S31N; ▵, M2del29-31; ○, wild type.
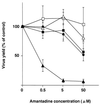
FIG. 3
Amantadine sensitivity of M2 ion channel mutants. The mutant and wild-type WSN-UdM viruses were tested for plaque-forming capacity in MDCK cells in the presence of different concentrations of amantadine. Experiments were performed three times, with the results reported as means ± SD. ■, M2V27T; □, M2A30P; ●, M2S31N; ▵, M2del29-31; ▴, wild type.
FIG. 4
Schematic diagram of the chimeric M2 mutants and the M2 mutant lacking the TM and cytoplasmic domains. Each chimeric mutant was constructed by replacing the TM domain of M2 with that of the HA or NA, while ΔM2TMCYT was constructed by introducing two stop codons at the 3′ end of the M1 ORF, resulting in a mutant lacking both the TM and cytoplasmic domains.
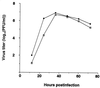
FIG. 5
Growth curves of M2HATM (□) and wild-type (●) WSN-UdM viruses. MDCK cells were infected with virus at an MOI of 0.001. At the indicated times after infection, the virus titer in the supernatant was determined. The values are means of triplicate experiments. The SD is less than 0.42 for each sample.
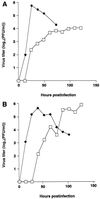
FIG. 6
Growth curves of ΔM2TMCYT (□) and wild-type (⧫) WSN-UdM viruses. MDCK cells were infected with virus at an MOI of 0.01 and incubated at 37°C (A) or 33°C (B). At the indicated times after infection, the virus titer in the supernatant was determined. The values are means of triplicate experiments. The SD is less than 0.40 for each sample.
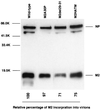
FIG. 7
Incorporation of M2 mutants into influenza virions. Purified viruses were lysed in sample buffer. Viral proteins were treated with 2-mercaptoethanol, separated by SDS–15% PAGE, transferred to a polyvinylidene difluoride membrane, and detected with the 14C2 anti-M2 monoclonal antibody and anti-WSN NP monoclonal antibody. Molecular masses of the marker proteins are shown on the left (in kilodaltons [K]).
Similar articles
- Influenza a virus M2 ion channel activity is essential for efficient replication in tissue culture.
Takeda M, Pekosz A, Shuck K, Pinto LH, Lamb RA. Takeda M, et al. J Virol. 2002 Feb;76(3):1391-9. doi: 10.1128/jvi.76.3.1391-1399.2002. J Virol. 2002. PMID: 11773413 Free PMC article. - The cysteine residues of the M2 protein are not required for influenza A virus replication.
Castrucci MR, Hughes M, Calzoletti L, Donatelli I, Wells K, Takada A, Kawaoka Y. Castrucci MR, et al. Virology. 1997 Nov 10;238(1):128-34. doi: 10.1006/viro.1997.8809. Virology. 1997. PMID: 9375016 - Direct measurement of the influenza A virus M2 protein ion channel activity in mammalian cells.
Wang C, Lamb RA, Pinto LH. Wang C, et al. Virology. 1994 Nov 15;205(1):133-40. doi: 10.1006/viro.1994.1628. Virology. 1994. PMID: 7526533 - [Structure and function of the influenza virus M2 ion channel protein].
Sakaguchi T. Sakaguchi T. Nihon Rinsho. 1997 Oct;55(10):2587-92. Nihon Rinsho. 1997. PMID: 9360376 Review. Japanese. - Controlling influenza virus replication by inhibiting its proton channel.
Pinto LH, Lamb RA. Pinto LH, et al. Mol Biosyst. 2007 Jan;3(1):18-23. doi: 10.1039/b611613m. Epub 2006 Nov 22. Mol Biosyst. 2007. PMID: 17216051 Review.
Cited by
- The M2 proteins of bat influenza A viruses reveal atypical features compared to conventional M2 proteins.
Thompson D, Cismaru CV, Rougier JS, Schwemmle M, Zimmer G. Thompson D, et al. J Virol. 2023 Aug 31;97(8):e0038823. doi: 10.1128/jvi.00388-23. Epub 2023 Aug 4. J Virol. 2023. PMID: 37540019 Free PMC article. - The impact of the suppression of highly connected protein interactions on the corona virus infection.
Torres F, Kiwi M, Schuller IK. Torres F, et al. Sci Rep. 2022 Jun 2;12(1):9188. doi: 10.1038/s41598-022-13373-0. Sci Rep. 2022. PMID: 35654986 Free PMC article. - Antigen modifications improve nucleoside-modified mRNA-based influenza virus vaccines in mice.
Freyn AW, Pine M, Rosado VC, Benz M, Muramatsu H, Beattie M, Tam YK, Krammer F, Palese P, Nachbagauer R, McMahon M, Pardi N. Freyn AW, et al. Mol Ther Methods Clin Dev. 2021 Jun 12;22:84-95. doi: 10.1016/j.omtm.2021.06.003. eCollection 2021 Sep 10. Mol Ther Methods Clin Dev. 2021. PMID: 34485597 Free PMC article. - Key Amino Acids of M1-41 and M2-27 Determine Growth and Pathogenicity of Chimeric H17 Bat Influenza Virus in Cells and in Mice.
Yang J, Zhang P, Huang M, Qiao S, Liu Q, Chen H, Teng Q, Li X, Zhang Z, Yan D, Sun H, Li Z. Yang J, et al. J Virol. 2021 Sep 9;95(19):e0101921. doi: 10.1128/JVI.01019-21. Epub 2021 Sep 9. J Virol. 2021. PMID: 34287044 Free PMC article. - Accessory Gene Products of Influenza A Virus.
Pinto RM, Lycett S, Gaunt E, Digard P. Pinto RM, et al. Cold Spring Harb Perspect Med. 2021 Dec 1;11(12):a038380. doi: 10.1101/cshperspect.a038380. Cold Spring Harb Perspect Med. 2021. PMID: 32988983 Free PMC article. Review.
References
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J D, editors. Molecular biology of the cell. New York, N.Y: Garland Publishing, Inc.; 1994.
- Appleyard G. Amantadine-resistance as a genetic marker for influenza viruses. J Gen Virol. 1977;36:249–255. - PubMed
- Cohen E A, Terwilliger E F, Sodroski J G, Haseltine W A. Identification of a protein encoded by the vpu gene of HIV-1. Nature. 1988;334:532–534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources