Direct binding of occupied urokinase receptor (uPAR) to LDL receptor-related protein is required for endocytosis of uPAR and regulation of cell surface urokinase activity - PubMed (original) (raw)
Direct binding of occupied urokinase receptor (uPAR) to LDL receptor-related protein is required for endocytosis of uPAR and regulation of cell surface urokinase activity
R P Czekay et al. Mol Biol Cell. 2001 May.
Free PMC article
Abstract
Low-density lipoprotein receptor-related protein (LRP) mediates internalization of urokinase:plasminogen activator inhibitor complexes (uPA:PAI-1) and the urokinase receptor (uPAR). Here we investigated whether direct interaction between uPAR, a glycosyl-phosphatidylinositol-anchored protein, and LRP, a transmembrane receptor, is required for clearance of uPA:PAI-1, regeneration of unoccupied uPAR, activation of plasminogen, and the ability of HT1080 cells to invade extracellular matrix. We found that in the absence of uPA:PAI-1, uPAR is randomly distributed along the plasma membrane, whereas uPA:PAI-1 promotes formation of uPAR-LRP complexes and initiates redistribution of occupied uPAR to clathrin-coated pits. uPAR-LRP complexes are endocytosed via clathrin-coated vesicles and traffic together to early endosomes (EE) because they can be coimmunoprecipitated from immunoisolated EE, and internalization is blocked by depletion of intracellular K(+). Direct binding of domain 3 (D3) of uPAR to LRP is required for clearance of uPA-PAI-1-occupied uPAR because internalization is blocked by incubation with recombinant D3. Moreover, uPA-dependent plasmin generation and the ability of HT1080 cells to migrate through Matrigel-coated invasion chambers are also inhibited in the presence of D3. These results demonstrate that GPI-anchored uPAR is endocytosed by piggybacking on LRP and that direct binding of occupied uPAR to LRP is essential for internalization of occupied uPAR, regeneration of unoccupied uPAR, plasmin generation, and invasion and migration through extracellular matrix.
Figures
Figure 1
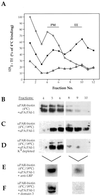
Expression of LRP by HT1080 cells. (A) LRP on HT1080 cells shows a high affinity for α2M (apparent_K_D = 0.75 nM). HT1080 cells were incubated with 125I- α2M (2 nM), an LRP-specific ligand, at 4°C in the presence or absence of varying amounts of unlabeled α2M (0.06–40.0 nM). Cell-associated radioactivity was quantitated by gamma counting, normalized to total cell protein, and the affinity was calculated as described in MATERIALS AND METHODS. (Inset) LRP immunoprecipitated from HT1080 cells comigrates with LRP from NRK cells. HT1080 cells and NRK cells (used as control) and cell lysates were radioiodinated and processed for immunoprecipitation with anti-LRP mAb (11H4). (B) HT1080 cells take up and degrade 125I-α2M linearly up to 8 h. Cells were incubated at 37°C with125I-α2M (2 nM) for 8 h. TCA-soluble radioactivity released into the incubation medium was used to quantify the amount of ligand degraded as described in MATERIALS AND METHODS.
Figure 2
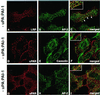
Distribution of LRP and uPAR on the surface of HT-1080 cells at steady state. (A–C) Double labeling showing significant overlap of LRP (A) and AP-2 (B) by immunofluorescence. (D–F) Little or no overlap is seen between uPAR (D) and caveolin (E). (G–I) Similarly, there is little or no overlap between uPAR (G) and AP-2 (H). Cells were incubated with polyclonal anti-uPAR (3936) or anti-LRP mAb (456) at 4°C, fixed with 2% formaldehyde, permeabilized with Triton X-100 and subsequently incubated with anti-AP-2 (mAb) or anti-caveolin (chicken), followed by detection with appropriate secondary antibodies.
Figure 3
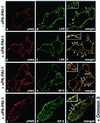
Distribution of uPAR in the presence of uPA:PAI-1 complexes and D3. (A–C) In HT1080 cells incubated in the absence of uPA:PAI:1 complexes there is little overlap in staining for LRP (A) and uPAR (B). (D–E) In the presence of uPA:PAI-1 overlap in the staining for uPAR (D) and LRP (E) is dramatically increased. There is also increased overlap in staining for uPAR (G) and AP-2 (H), indicating redistribution of uPAR to clathrin-coated pits. (J and K) Redistribution of uPAR to clathrin-coated pits in the presence of uPA:PAI-1 is not affected by the presence of D3. HT1080 cells were acid-washed, incubated at 4°C in the absence or presence of uPA:PAI-1 complexes and D3 (J–L), and processed for immunofluorescence as described in Figure 2 legend.
Figure 4
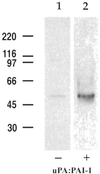
LRP and uPAR form detergent-stable complexes at the surface of HT1080 cells in the presence of uPA:PAI-1. In the absence of uPA:PAI-1 (lane 1), only traces of uPAR are detected in immunoprecipitates obtained with anti-LRP, whereas in the presence of uPA:PAI-1 immunoprecipitates contain considerable uPAR (lane 2). HT1080 cells were surface radioiodinated, incubated in the presence or absence of uPA:PAI-1, and processed for immunoprecipitation with anti-LRP mAb (11H4). Precipitated proteins were subsequently immunoblotted with anti-uPAR (465).
Figure 5
Direct binding of soluble uPAR to LRP via D3 of uPAR. (A) Considerable 125I-uPAR1-274 can be detected in anti-LRP immunoprecipitates (lane 2) after cross-linking. The amount of cross-linked 125I-uPAR1-274 is reduced (80–90%) in the presence of unlabeled uPAR1-274 (lane 3) and D3 (lane 5). D2 (lane 4) has no significant effect on uPAR1-274 binding to LRP. Acid-washed cells were incubated with 25 nM recombinant 125I-uPAR1-274 at 4°C in the presence (+) or absence (−) of 2.5 μM unlabeled uPAR1-274, or 1.25 μM recombinant D2, or D3 followed by cross-linking with DTSSP (lanes 2–5) and immunoprecipitation with anti-LRP mAb (11H4). Immunoprecipitated proteins were processed for autoradiography and densitometry. (B) Binding of125I-uPAR1-274 to affinity-purified LRP (lane 1) is reduced by >85% in the presence of unlabeled uPAR1-274 (lane 2) or D3 (lane 4). D2 (lane 3) has no effect on 125I-uPAR1-274 binding to LRP. In addition, 125I-D3 binds to affinity-purified LRP (lane 5) and the binding is reduced by >95% in the presence of unlabeled D3 (lane 6). Purified LRP was incubated with125I-uPAR1-274 (100 nM) or 125I-D3 (50 nM) at 4°C in the absence or presence of unlabeled uPAR1-274 (4 μM), D2 (1.5 μM), or D3 (1.5 μM). Immunoprecipitated proteins were processed for autoradiography and densitometry. (C) Binding of 125I-uPA:PAI-1 to recombinant uPAR1-274 (lane 1) is not affected by D3 (lane 2). uPAR1-274 (2 nM) was incubated with125I-uPA:PAI-1 (10 nM) in PBS in the presence or absence of D3 (100 nM) and processed for immunoprecipitation with anti-uPAR (465). Samples were processed for autoradiography and densitometry.
Figure 6
Internalization of uPAR requires binding to LRP. (A) In cells incubated with 125I-Tf at 4°C (□), Tf peaks in fractions 4–6, indicating the location of PM. After incubation at 18°C (▪), 125I-Tf peaks in fractions 8–10 where it defines the location of EE. In cells subjected to K+ depletion (▵), uptake of 125I-Tf is blocked, because no 125I-Tf cosedimented with EE. (B) In cells incubated at 4°C with uPA:PAI-1, biotinylated uPAR sediments in PM fractions 4–6. (C) After 60-min incubation at 18°C, the distribution of a substantial fraction of the biotinylated uPAR shifts to EE fractions (8–10). (D) After K+ depletion and incubation at 18°C the majority of the biotinylated uPAR is found in PM fractions (4–6), and little or no uPAR is detected in EE fractions (8–10), indicating that internalization of uPAR is blocked. After incubation for 60 min at 18°C in the presence of 200 μg/ml anti-LRP (456) (E), the majority of the biotinylated uPAR remains associated with PM fractions. (F) In the presence of 1.25 μM D3, virtually all detectable, biotinylated uPAR (∼95%) is found in PM fractions after incubation at 18°C. HT1080 cells were acid-washed, surface biotinylated, and incubated with either 125I-Tf (200 ng/ml) or uPA:PAI-1 complexes (50 nM) at 4°C (60 min) or for 4°C (60 min) followed by 18°C (60 min). In some cases D3 (1.25 μM) or anti-LRP antibodies (456; 200 μM/ml) were added or intracellular K+ was depleted as described in MATERIALS AND METHODS. Postnuclear supernatants were fractionated on Percoll gradients, and fractions were processed for gamma counting of 125I-Tf or for immunoprecipitation of uPAR and by detection of the biotinylated, cell surface pool of uPAR by blotting with HRP-coupled avidin. In E and F, PM fractions (4–6) and EE fractions (8–10), were pooled before analysis.
Figure 7
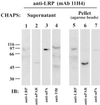
Presence of LRP and uPAR complexes in EE. The majority of the uPAR (lane 6) coprecipitates with LRP (lane 5) and is found in the pellet, which contains only trace amounts of uPA: PAI-1 (lane 7). The majority of the uPA:PAI-1 complexes (lane 3) and Tf receptor (lane 4) do not coprecipitate with LRP and are found in the supernatant, which contains only traces of LRP (lane 1) and uPAR (lane 2). Thus, uPAR and LRP form a complex that can be coprecipitated from EE. uPA:PAI-1 was bound to biotinylated HT1080 cells at 4°C, and the cells were fractionated as in Figure 6. EE fractions were pooled and LRP-containing vesicles were immunoisolated on mAb 11H4 (against the cytoplasmic tail of LRP) bound to protein G-agarose beads. Beads were treated with 10 mM CHAPS, and the proteins released into the supernatant and those remaining bound to the beads were analyzed by immunoblotting with anti-LRP (1073), anti-uPAR (465), anti-uPA, and anti-Tf receptor.
Figure 8
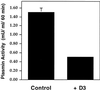
Inhibition of uPAR/LRP interaction reduces plasminogen activation. Plasmin activity at the cell surface of HT1080 cells is reduced from 1.5 mU (control) to 0.5 mU/ml/60 min when cells are incubated in the presence of D3. Acid-washed cells were incubated sequentially for 1 h at 4°C and 1.5 h at 37°C with uPA:PAI-1 (10 nM) in the presence or absence of D3 (5 μg/ml) and subsequently incubated at 4°C with proteolytically active uPA (2 nM) for 1 h in the presence of anti-LRP antibodies (456; 200 μg/ml). Plasminogen (0.2 μM) was bound to the cell surface at 4°C, and uPA-dependent plasmin generation was calculated as absorbance change at 405 nm over 60 min using Chromozym PL as a plasmin substrate.
Figure 9
Blocking uPAR/LRP interaction inhibits migration of HT1080 cells through Matrigel-coated filters. Cells (5 × 105) were seeded onto the top of Matrigel-coated filter membranes and incubated at 37°C in the presence or absence of uPAR1-274 (2.5 μM), D3 (1.25 μM), or D3 in combination with active uPA (8 nM). Cells that migrated through the filters and appeared at the under side of the filters were counted after 36 h of incubation. In the presence of uPAR1-274 and D3 the number of invading cells is decreased to 46.5 and 7.5% of control, respectively. Coincubation of cells with D3 and active uPA restores the ability of the cells to migrate to near control levels.
Figure 10
Model depicting proposed events in the interaction and trafficking of uPAR and LRP. (A) Active uPA binds to uPAR on the PM. 1) PAI-1 binds to and inhibits uPA. 2) uPA:PAI-1 bridges occupied uPAR and LRP, which leads to relocation of occupied uPAR into clathrin-coated pits and promotes a stable interaction between D3 of uPAR and LRP. 3) These quaternary complexes are internalized via clathrin-coated pits and delivered to EE. 4) uPA:PAI-1 dissociates from uPAR and LRP in EE and traffics through late endosomes (LE) to lysosomes for degradation. 5) Unoccupied uPAR and LRP also dissociate and 6) return to the cell surface via recycling vesicles (RV). This makes available unoccupied uPAR capable of binding pro-uPA. 7) Presence of active uPA at the cell surface catalyzes the generation of plasmin, which can degrade extracellular matrix. (B) When binding of occupied uPAR to LRP is prevented, uPA:PAI-1 bridges uPAR and LRP and occupied uPAR moves into clathrin-coated pits. However, clearance of occupied uPAR is blocked (8) and occupied inactive uPAR accumulates on the cell surface. As a consequence, generation of plasmin and degradation and invasion of extracellular matrix are decreased.
Similar articles
- alpha-2 Macroglobulin receptor/Ldl receptor-related protein(Lrp)-dependent internalization of the urokinase receptor.
Conese M, Nykjaer A, Petersen CM, Cremona O, Pardi R, Andreasen PA, Gliemann J, Christensen EI, Blasi F. Conese M, et al. J Cell Biol. 1995 Dec;131(6 Pt 1):1609-22. doi: 10.1083/jcb.131.6.1609. J Cell Biol. 1995. PMID: 8522616 Free PMC article. - Urokinase/urokinase receptor system: internalization/degradation of urokinase-serpin complexes: mechanism and regulation.
Conese M, Blasi F. Conese M, et al. Biol Chem Hoppe Seyler. 1995 Mar;376(3):143-55. Biol Chem Hoppe Seyler. 1995. PMID: 7612191 Review. - Structure, function and expression on blood and bone marrow cells of the urokinase-type plasminogen activator receptor, uPAR.
Plesner T, Behrendt N, Ploug M. Plesner T, et al. Stem Cells. 1997;15(6):398-408. doi: 10.1002/stem.150398. Stem Cells. 1997. PMID: 9402652 Review.
Cited by
- Packaging of supplemented urokinase into alpha granules of in vitro-grown megakaryocytes for targeted nascent clot lysis.
Poncz M, Zaitsev SV, Ahn H, Kowalska MA, Bdeir K, Dergilev KV, Ivanciu L, Camire RM, Cines DB, Stepanova V. Poncz M, et al. Blood Adv. 2024 Jul 23;8(14):3798-3809. doi: 10.1182/bloodadvances.2024012835. Blood Adv. 2024. PMID: 38805575 Free PMC article. - The uPA/uPAR System Orchestrates the Inflammatory Response, Vascular Homeostasis, and Immune System in Fibrosis Progression.
Kanno Y. Kanno Y. Int J Mol Sci. 2023 Jan 16;24(2):1796. doi: 10.3390/ijms24021796. Int J Mol Sci. 2023. PMID: 36675310 Free PMC article. Review. - Simulated in vitro hypoxic conditions from psoriatic arthritis cartilage change plasminogen activating system urokinase and serpine functionality. Reversal of antiapoptotic protection suggests common homeostatic buffering.
Nohawica M, Nowak-Terpilowska A, Adamska K, Wyganowska-Swiatkowska M. Nohawica M, et al. Postepy Dermatol Alergol. 2022 Oct;39(5):944-952. doi: 10.5114/ada.2022.113405. Epub 2022 Feb 8. Postepy Dermatol Alergol. 2022. PMID: 36457693 Free PMC article. - Modulation of Cellular Function by the Urokinase Receptor Signalling: A Mechanistic View.
Alfano D, Franco P, Stoppelli MP. Alfano D, et al. Front Cell Dev Biol. 2022 Apr 8;10:818616. doi: 10.3389/fcell.2022.818616. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35493073 Free PMC article. Review. - Urokinase-type plasminogen activator receptor (uPAR) as a therapeutic target in cancer.
Zhai BT, Tian H, Sun J, Zou JB, Zhang XF, Cheng JX, Shi YJ, Fan Y, Guo DY. Zhai BT, et al. J Transl Med. 2022 Mar 18;20(1):135. doi: 10.1186/s12967-022-03329-3. J Transl Med. 2022. PMID: 35303878 Free PMC article. Review.
References
- Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. - PubMed
- Anderson RGW. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. - PubMed
- Andreasen PA, Sottrup-Jensen L, Kjøller L, Nykjaer A, Moestrup SK, Petersen CM, Gliemann J. Receptor-mediated endocytosis of plasminogen activators and activator/inhibitor complexes. FEBS Lett. 1994;338:239–245. - PubMed
- Behrendt N, Ploug M, Patthy L, Houen G, Blasi F, Dano K. The ligand-binding domain of the cell surface receptor for urokinase-type plasminogen activator. J Biol Chem. 1991;266:7842–7847. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous