Human placental cytotrophoblasts attract monocytes and CD56(bright) natural killer cells via the actions of monocyte inflammatory protein 1alpha - PubMed (original) (raw)
Human placental cytotrophoblasts attract monocytes and CD56(bright) natural killer cells via the actions of monocyte inflammatory protein 1alpha
P M Drake et al. J Exp Med. 2001.
Abstract
During human pregnancy, the specialized epithelial cells of the placenta (cytotrophoblasts) come into direct contact with immune cells in several locations. In the fetal compartment of the placenta, cytotrophoblast stem cells lie adjacent to macrophages (Hofbauer cells) that reside within the chorionic villus stroma. At sites of placental attachment to the mother, invasive cytotrophoblasts encounter specialized maternal natural killer (NK) cells (CD56(bright)), macrophages, and T cells that accumulate within the uterine wall during pregnancy. Here we tested the hypothesis that fetal cytotrophoblasts can direct the migration of these maternal immune cells. First, we assayed the chemotactic activity of cytotrophoblast conditioned medium samples, using human peripheral blood mononuclear cells as targets. The placental samples preferentially attracted NK cells (both CD56(dim) and CD56(bright)), monocytes, and T cells, suggesting that our hypothesis was correct. A screen to identify chemokine activity through the induction of a Ca(2)+ flux in cells transfected with individual chemokine receptors suggested that cytotrophoblasts secreted monocyte inflammatory protein (MIP)-1alpha. This was confirmed by localizing the corresponding mRNA and protein, both in vitro and in vivo. MIP-1alpha protein in conditioned medium was further characterized by immunoblotting and enzyme-linked immunosorbent assay. Immunodepletion of MIP-1alpha from cytotrophoblast conditioned medium showed that this chemokine was responsible for a significant portion of the induced monocyte and CD56(bright) NK cell chemotaxis. These data suggest the specific conclusion that cytotrophoblasts can attract monocytes and CD56(bright) NK cells by producing MIP-1alpha and the more general hypothesis that these cells may organize and act on leukocytes at the maternal-fetal interface.
Figures
Figure 1
The cellular architecture of the human maternal–fetal interface where fetal placental cells (cytotrophoblasts) are in close proximity to maternal leukocytes that reside within the uterine wall. The chorionic villus is the basic structural unit of the placenta. A subset, termed anchoring chorionic villi (AV), establishes physical connections between the fetus and the mother. Anchoring villi form when cytotrophoblast stem cells detach from their basement membrane and form a column of nonpolarized mononuclear cells that invade the uterus. These invasive cytotrophoblasts rapidly traverse most of the uterine parenchyma, where they encounter numerous maternal immune cells, predominantly CD56bright NK cells, but also macrophages and T cells. Invasive cytotrophoblasts target uterine spiral arterioles for invasion. They remodel these vessels by replacing the endothelial lining and destroying the muscular wall. Thus, cytotrophoblasts are in direct contact with both maternal peripheral blood (sites marked 1) and decidual leukocytes (sites marked 2). FV, floating villi.
Figure 2
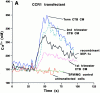
Cytotrophoblast CM signals through MIP-1α receptors. Cytotrophoblast (CTB) CM was screened for its ability to induce a calcium flux in reporter cells transfected with individual chemokine receptors. Negative controls included exposure of the transfected cells to serum-free control medium incubated with Matrigel in the absence of cells (SFM/MG) and addition of cytotrophoblast CM to the corresponding untransfected parental cell line. Recombinant chemokines were used as positive controls. Concentrated cytotrophoblast CM induced a Ca2+ flux in cells expressing CCR1 (A) and CCR5 (B), but not CCR2, CCR3, or CX3CR1 (data not shown).
Figure 2
Cytotrophoblast CM signals through MIP-1α receptors. Cytotrophoblast (CTB) CM was screened for its ability to induce a calcium flux in reporter cells transfected with individual chemokine receptors. Negative controls included exposure of the transfected cells to serum-free control medium incubated with Matrigel in the absence of cells (SFM/MG) and addition of cytotrophoblast CM to the corresponding untransfected parental cell line. Recombinant chemokines were used as positive controls. Concentrated cytotrophoblast CM induced a Ca2+ flux in cells expressing CCR1 (A) and CCR5 (B), but not CCR2, CCR3, or CX3CR1 (data not shown).
Figure 6
Invasive cytotrophoblasts express MIP-1α mRNA in vivo. In situ hybridization on tissue sections of the maternal–fetal interface at term demonstrates the presence of MIP-1α mRNA in cytotrophoblasts within the uterine wall. (A) Bright field micrograph of a histological section that was stained with hematoxylin and eosin (HE). Clusters of cytotrophoblasts are easily seen within a loose meshwork of extracellular matrix components. (B) Dark field micrograph of the same section. White dots indicate signal from 35S-labeled MIP-1α antisense probe (8-wk exposure). This signal is absent in an adjacent section (C) that was incubated with sense probe as a negative control. AV, anchoring villus.
Figure 3
In situ hybridization demonstrates that cultured cytotrophoblasts synthesize MIP-1α mRNA in vitro. (A) Bright field micrograph of a histological section of cultured cytotrophoblast cells that was stained with hematoxylin and eosin (HE). Dark field micrographs of control sense probes (B) were negative, while experimental antisense probes (C) revealed cytotrophoblast expression of MIP-1α mRNA, detected as white dots (4 wk exposure). Immunohistochemistry on adjacent sections demonstrated positive staining for the cytotrophoblast marker cytokeratin (CK) (D) and the absence of staining for the macrophage marker CD45Rb (E).
Figure 4
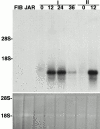
Northern blot hybridization shows that cytotrophoblasts modulate MIP-1α mRNA expression in culture. Top panel: Cytotrophoblast expression of MIP-1α mRNA was quantified as a function of differentiation/invasion in vitro. Total RNA was isolated from first trimester placental fibroblasts (FIB) from the choriocarcinoma cell line JAR, and from first (I) and second (II) trimester cytotrophoblasts either immediately after isolation (0 h) or after culturing the cells for the hours indicated. Northern blot analysis was performed using an MIP-1α–specific probe. Prior to culture, first and second trimester cells expressed very low levels of this mRNA, but its expression was upregulated by ∼30-fold after 12 h in culture. Neither placental fibroblasts nor the JAR cell line expressed MIP-1α mRNA. Bottom panel: Acridine orange staining of the gel before transfer demonstrated equal loading of RNA.
Figure 5
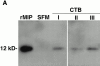
Cytotrophoblasts secrete MIP-1α protein in vitro_._ (A) Immunoblotting with an MIP-1α–specific polyclonal IgG. This antibody reacted with a single band of the expected size (12 kD) in a sample of recombinant MIP-1α (rMIP). Immunoreactive bands of the same estimated molecular mass were present in concentrated CM samples of cytotrophoblasts isolated from first (I), second (II), and third (III) trimester placentas, but not the control SFM. (B) MIP-1α concentrations in cytotrophoblast CM as assessed by ELISA. CM was harvested from first trimester, second trimester, and term cells that were cultured for 12–48 h. Levels of this chemokine detected in individual samples ranged from 1.2–82 ng/ml. The five sets of connected data points show MIP-1α levels in medium samples from the same culture over time, demonstrating that the protein accumulated in the medium during the course of the experiment. As expected, MIP-1α was not detected in control SFM/MG or placental fibroblast CM (data not shown).
Figure 5
Cytotrophoblasts secrete MIP-1α protein in vitro_._ (A) Immunoblotting with an MIP-1α–specific polyclonal IgG. This antibody reacted with a single band of the expected size (12 kD) in a sample of recombinant MIP-1α (rMIP). Immunoreactive bands of the same estimated molecular mass were present in concentrated CM samples of cytotrophoblasts isolated from first (I), second (II), and third (III) trimester placentas, but not the control SFM. (B) MIP-1α concentrations in cytotrophoblast CM as assessed by ELISA. CM was harvested from first trimester, second trimester, and term cells that were cultured for 12–48 h. Levels of this chemokine detected in individual samples ranged from 1.2–82 ng/ml. The five sets of connected data points show MIP-1α levels in medium samples from the same culture over time, demonstrating that the protein accumulated in the medium during the course of the experiment. As expected, MIP-1α was not detected in control SFM/MG or placental fibroblast CM (data not shown).
Figure 8
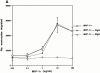
The effects of recombinant MIP-1α on PBMC chemotaxis. (A–C) Results of a representative experiment. (A) Monocyte chemotaxis toward a dilution series of recombinant MIP-1α (rMIP-1α) peaked at a concentration of 10 ng/ml. Addition of a neutralizing anti–MIP-1α IgG (NIgG) returned migration to baseline levels, and a control IgG antibody (CIgG) added at the same concentration had no effect. (B) CD56bright and (C) CD56dim NK cells also responded to recombinant MIP-1α, but their migration did not peak within the range of concentrations tested. Addition of anti–MIP-1α reduced NK cell migration to baseline.
Figure 8
The effects of recombinant MIP-1α on PBMC chemotaxis. (A–C) Results of a representative experiment. (A) Monocyte chemotaxis toward a dilution series of recombinant MIP-1α (rMIP-1α) peaked at a concentration of 10 ng/ml. Addition of a neutralizing anti–MIP-1α IgG (NIgG) returned migration to baseline levels, and a control IgG antibody (CIgG) added at the same concentration had no effect. (B) CD56bright and (C) CD56dim NK cells also responded to recombinant MIP-1α, but their migration did not peak within the range of concentrations tested. Addition of anti–MIP-1α reduced NK cell migration to baseline.
Figure 8
The effects of recombinant MIP-1α on PBMC chemotaxis. (A–C) Results of a representative experiment. (A) Monocyte chemotaxis toward a dilution series of recombinant MIP-1α (rMIP-1α) peaked at a concentration of 10 ng/ml. Addition of a neutralizing anti–MIP-1α IgG (NIgG) returned migration to baseline levels, and a control IgG antibody (CIgG) added at the same concentration had no effect. (B) CD56bright and (C) CD56dim NK cells also responded to recombinant MIP-1α, but their migration did not peak within the range of concentrations tested. Addition of anti–MIP-1α reduced NK cell migration to baseline.
Figure 7
Invasive cytotrophoblasts express protein MIP-1α in vivo. Immunohistochemistry on tissue sections of the maternal–fetal interface identifies cytokeratin (CK)-positive invasive cytotrophoblasts within the uterine wall (A). Staining of an adjacent section reveals that these invasive cytotrophoblasts express MIP-1α protein (B). The specificity of the MIP-1α pattern was established by staining with normal goat serum as a negative control. Other specimens were exposed to (C) an anticytokeratin antibody, which demonstrated the cytotrophoblasts within the tissue, and (D) to the control serum, which did not yield a signal. NGS, normal goat serum; V, chorionic villus.
Figure 9
Cytotrophoblast CM attracts monocytes and CD56bright NK cells via the action of MIP-1α. Panels A–C depict the results of a representative experiment. MIP-1α levels in cytotrophoblast CM samples were determined by ELISA. The chemoattractant activity of cytotrophoblast CM that contained from 0.01 to 100 ng/ml MIP-1α is summarized in A. In all experiments, the migration of cells toward SFM that was incubated with Matrigel alone (SFM/MG) was used to assess basal migration. Monocyte movement toward the CM peaked at 10 ng/ml of MIP-1α, while NK cell and T cell migration was still increasing at the highest concentration of CM (chemokine) tested. (B and C) Addition of a neutralizing anti–MIP-1α IgG (NIgG) significantly reduced the migration of PBMCs toward cytotrophoblast CM. (B) At its peak, monocyte migration was reduced by 44.2 ± 10.0% (mean ± SD) as compared to the addition of a control IgG (CIgG). (C) Chemotaxis of CD56bright NK cells was diminished by 66.8 ± 20.0% as compared to the control IgG. Neither CD56dim NK cell nor T cell migration was affected by neutralization of MIP-1α activity (data not shown).
Figure 9
Cytotrophoblast CM attracts monocytes and CD56bright NK cells via the action of MIP-1α. Panels A–C depict the results of a representative experiment. MIP-1α levels in cytotrophoblast CM samples were determined by ELISA. The chemoattractant activity of cytotrophoblast CM that contained from 0.01 to 100 ng/ml MIP-1α is summarized in A. In all experiments, the migration of cells toward SFM that was incubated with Matrigel alone (SFM/MG) was used to assess basal migration. Monocyte movement toward the CM peaked at 10 ng/ml of MIP-1α, while NK cell and T cell migration was still increasing at the highest concentration of CM (chemokine) tested. (B and C) Addition of a neutralizing anti–MIP-1α IgG (NIgG) significantly reduced the migration of PBMCs toward cytotrophoblast CM. (B) At its peak, monocyte migration was reduced by 44.2 ± 10.0% (mean ± SD) as compared to the addition of a control IgG (CIgG). (C) Chemotaxis of CD56bright NK cells was diminished by 66.8 ± 20.0% as compared to the control IgG. Neither CD56dim NK cell nor T cell migration was affected by neutralization of MIP-1α activity (data not shown).
Figure 9
Cytotrophoblast CM attracts monocytes and CD56bright NK cells via the action of MIP-1α. Panels A–C depict the results of a representative experiment. MIP-1α levels in cytotrophoblast CM samples were determined by ELISA. The chemoattractant activity of cytotrophoblast CM that contained from 0.01 to 100 ng/ml MIP-1α is summarized in A. In all experiments, the migration of cells toward SFM that was incubated with Matrigel alone (SFM/MG) was used to assess basal migration. Monocyte movement toward the CM peaked at 10 ng/ml of MIP-1α, while NK cell and T cell migration was still increasing at the highest concentration of CM (chemokine) tested. (B and C) Addition of a neutralizing anti–MIP-1α IgG (NIgG) significantly reduced the migration of PBMCs toward cytotrophoblast CM. (B) At its peak, monocyte migration was reduced by 44.2 ± 10.0% (mean ± SD) as compared to the addition of a control IgG (CIgG). (C) Chemotaxis of CD56bright NK cells was diminished by 66.8 ± 20.0% as compared to the control IgG. Neither CD56dim NK cell nor T cell migration was affected by neutralization of MIP-1α activity (data not shown).
Similar articles
- [The expression of chemokine receptors in CD56(bright) CD16- natural killer cells and the mechanism of their recruitment in decidua].
Wu X, Li DJ, Yuan MM, Zhu Y, Wang MY. Wu X, et al. Zhonghua Yi Xue Za Zhi. 2004 Jun 17;84(12):1018-23. Zhonghua Yi Xue Za Zhi. 2004. PMID: 15312539 Chinese. - Chemokine and chemokine receptor expression by liver-derived dendritic cells: MIP-1alpha production is induced by bacterial lipopolysaccharide and interaction with allogeneic T cells.
Drakes ML, Zahorchak AF, Takayama T, Lu L, Thomson AW. Drakes ML, et al. Transpl Immunol. 2000 Mar;8(1):17-29. doi: 10.1016/s0966-3274(00)00002-2. Transpl Immunol. 2000. PMID: 10834607 - Role of chemokines in the biology of natural killer cells.
Maghazachi AA. Maghazachi AA. Curr Top Microbiol Immunol. 2010;341:37-58. doi: 10.1007/82_2010_20. Curr Top Microbiol Immunol. 2010. PMID: 20369317 Review. - Immunological relationship between the mother and the fetus.
Szekeres-Bartho J. Szekeres-Bartho J. Int Rev Immunol. 2002 Nov-Dec;21(6):471-95. doi: 10.1080/08830180215017. Int Rev Immunol. 2002. PMID: 12650238 Review.
Cited by
- Reduced ability of newborns to produce CCL3 is associated with increased susceptibility to perinatal human immunodeficiency virus 1 transmission.
Meddows-Taylor S, Donninger SL, Paximadis M, Schramm DB, Anthony FS, Gray GE, Kuhn L, Tiemessen CT. Meddows-Taylor S, et al. J Gen Virol. 2006 Jul;87(Pt 7):2055-2065. doi: 10.1099/vir.0.81709-0. J Gen Virol. 2006. PMID: 16760409 Free PMC article. - Manifestations of immune tolerance in the human female reproductive tract.
Clark GF, Schust DJ. Clark GF, et al. Front Immunol. 2013 Feb 13;4:26. doi: 10.3389/fimmu.2013.00026. eCollection 2013. Front Immunol. 2013. PMID: 23407606 Free PMC article. - Killer-cell immunoglobulin-like receptor/human leukocyte antigen-C combination and 'great obstetrical syndromes' (Review).
Yang X, Meng T. Yang X, et al. Exp Ther Med. 2021 Oct;22(4):1178. doi: 10.3892/etm.2021.10612. Epub 2021 Aug 13. Exp Ther Med. 2021. PMID: 34504623 Free PMC article. Review. - Chemokine expression and function at the human maternal-fetal interface.
Drake PM, Red-Horse K, Fisher SJ. Drake PM, et al. Rev Endocr Metab Disord. 2002 May;3(2):159-65. doi: 10.1023/a:1015463130306. Rev Endocr Metab Disord. 2002. PMID: 12007293 Review. No abstract available. - IFN-γ induces aberrant CD49b⁺ NK cell recruitment through regulating CX3CL1: a novel mechanism by which IFN-γ provokes pregnancy failure.
Li ZY, Chao HH, Liu HY, Song ZH, Li LL, Zhang YJ, Yang Y, Peng JP. Li ZY, et al. Cell Death Dis. 2014 Nov 6;5(11):e1512. doi: 10.1038/cddis.2014.470. Cell Death Dis. 2014. PMID: 25375377 Free PMC article.
References
- Boyd J.G., Hamilton W.J. The Human Placenta 1970. Heffer; Cambridge, UK: pp. 646
- Brosens I., Dixon H.G. The anatomy of the maternal side of the placenta. J. Obstet. Gynaecol. Br. Commonw. 1966;73:357–363. - PubMed
- Cross J.C., Werb Z., Fisher S.J. Implantation and the placentakey pieces of the development puzzle. Science. 1994;266:1508–1518. - PubMed
- Damsky C.H., Fisher S.J. Trophoblast pseudo-vasculogenesisfaking it with endothelial adhesion receptors. Curr. Opin. Cell Biol. 1998;10:660–666. - PubMed
- Copeman J., Han R.N., Caniggia I., McMaster M., Fisher S.J., Cross J.C. Posttranscriptional regulation of human leukocyte antigen G during human extravillous cytotrophoblast differentiation. Biol. Reprod. 2000;62:1543–1550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous