The MER3 helicase involved in meiotic crossing over is stimulated by single-stranded DNA-binding proteins and unwinds DNA in the 3' to 5' direction - PubMed (original) (raw)
The MER3 helicase involved in meiotic crossing over is stimulated by single-stranded DNA-binding proteins and unwinds DNA in the 3' to 5' direction
T Nakagawa et al. J Biol Chem. 2001.
Abstract
The meiosis-specific MER3 protein of Saccharomyces cerevisiae is required for crossing over, which ensures faithful segregation of homologous chromosomes at the first meiotic division. The predicted sequence of the MER3 protein contains the seven motifs characteristic of the DExH-box type of DNA/RNA helicases. The purified MER3 protein is a DNA helicase, which can displace a 50-nucleotide fragment annealed to a single-stranded circular DNA. MER3 was found to have ATPase activity, which was stimulated either by single- or double-stranded DNA. The turnover rate, k(cat), of ATP hydrolysis was approximately 500/min in the presence of either DNA. MER3 was able to efficiently displace relatively long 631-nucleotide fragments from single-stranded circular DNA only in the presence of the S. cerevisiae single-stranded DNA-binding protein, RPA (replication protein A). It appears that RPA inhibits re-annealing of the single-stranded products of the MER3 helicase. The MER3 helicase was found to unwind DNA in the 3' to 5' direction relative to single-stranded regions in the DNA substrates. Possible roles for the MER3 helicase in meiotic crossing over are discussed.
Figures
Fig. 1. Poly (dA) and M13 RF DNA stimulate the MER3 ATPase activity
Reactions (20 µl each) containing different concentrations (0, 0.04, 0.1, 0.3, 0.6, 0.9, 1.2, 1.5, 1.8, and 2.1 µg/ml) of poly (dA) (A) or M13mp18 RF (B) were initiated by the addition of MER3, incubated at 30 °C for 15 min, and terminated by the addition of 2 µl of 0.5
m
EDTA, and the amount of ATP hydrolyzed was measured as described under “Materials and Methods.” C and D, the initial velocity of ATP hydrolysis in reactions (40 µl each) was measured at different concentrations of ATP (0.25, 0.33, 0.5, 1, and 2 m
m
) in the presence of 10 µg/ml poly (dA) (C) or M13mp18 RF DNA (D); the data are presented as a Lineweaver-Burk plot. The average of ATP hydrolyzed in three independent experiments is plotted, and the error bars show the standard deviation.
Fig. 2. Requirement of divalent cations for the MER3 ATPase activity
Reactions (20 µl each) were carried out as described in the legend to Fig. 1, A and B, except that EDTA was present at a final concentration of 0.1 m
m
in order to remove contaminating divalent cations; and then CaCl2, MgCl2, MnCl2, or ZnCl2 was added to a final concentration of 5 m
m
prior to the pre-incubation step. The amount of ATP hydrolyzed in the presence of poly (dA) (A) or M13 RF (B) is presented. The average of two independent experiments is shown, and the error bars indicate the standard deviation.
Fig. 3. The mer3G166D mutation impairs ATPase activity
A time course of ATP hydrolysis by the wild-type MER3 and mutant MER3GD proteins was performed as described under “Materials and Methods.” Reactions (40 µl each) were incubated for the indicated times in the presence of poly (dA) (A) or M13mp18 RF (B). The average of three independent experiments is presented, and the error bars indicate the standard deviation.
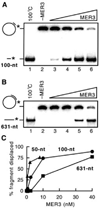
Fig. 4. Displacement of different length fragments by MER3 helicase
Displacement of 5′ -end-labeled fragments annealed to M13mp18 single-stranded circular DNA was examined as described under “Materials and Methods.” The reactions contained 1 µ
m
(in nucleotides) DNA substrates. 6 and 4% non-denaturing polyacrylamide gels were used for the detection of the 100- and 631-nt fragments, respectively. Increasing amounts (0, 0.625, 2.5, 10, and 40 n
m
) of MER3 were added to the reaction containing the 100-nt fragment substrate (A) or the 631-nt fragment substrate (B). The percentage of fragment displaced by the different amounts of MER3 is plotted (C). To obtain the percentage for the 100- and 631-nt fragments, the gels shown in A and B, respectively, were analyzed using a PhosphorImager. The percentage displacement for 50-nt fragment substrate was measured similarly except that the reactions contained 0, 0.5, 2, or 8 n
m
MER3.
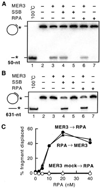
Fig. 5. RPA and SSB stimulate displacement of the 631-nt fragment
Displacement of 5′ -end-labeled fragments annealed to M13mp18 single-stranded circular DNA was examined in the presence or absence of 20 n
m
S. cerevisiae RPA or E. coli SSB, essentially as described under “Materials and Methods.” Reactions contained 1 µ
m
(in nucleotides) DNA substrates. Displacement of 50-nt (A) and 631-nt (B) fragments was analyzed in the presence of 0.2 and 5 n
m
MER3, respectively, and 8 and 4% non-denaturing polyacrylamide gels were used for the detection of 50- and 631-nt fragments, respectively. C, effect of increasing RPA concentration (0, 2.5, 5, 10, 20, and 40 n
m
) in reactions containing 1 µ
m
(in nucleotides) 631-nt fragment substrates. RPA was added to the reaction 5 min before (closed circles) or 2 min after (closed triangles) the addition of 5 n
m
MER3. The open circles indicate the reaction to which no MER3 was added.
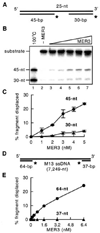
Fig. 6. Unwinding of DNA duplexes flanking a single-stranded region
A, the 100-nt DNA substrate containing annealed 5′ -end-labeled 45- and 30-nt fragments used to determine the polarity of MER3 helicase is illustrated. B, standard helicase reactions (20 µl each) containing 2 n
m
(in molecules) DNA substrate, illustrated in A, and 10 m
m
NaCl were started by the addition of 1, 2, 3, 4, or 5 n
m
MER3, and incubated at 30 °C for 30 min. The DNA product formed was monitored by electrophoresis through 10% polyacrylamide gels. C, the average value of the amount of 45- and 30-nt fragments displaced in three independent experiments is plotted. The error bars indicate the standard deviation. D, the DNA substrate containing an ~7,100-nt-long single-stranded region flanked by double-stranded regions containing end-labeled 64- and 37-nt fragments used to determine the polarity of MER3 helicase is illustrated. E, standard helicase reactions (20 µl each) containing 1 µ
m
(in nucleotides) DNA substrate having a long single-stranded region, illustrated in panel D, and 100 m
m
NaCl were started by the addition of 0.2, 0.4, 0.8, 1.6, 3.2, or 6.4 n
m
of MER3 and incubated at 30 °C for 30 min. The percentage of 64- or 37-nt fragment displaced is plotted.
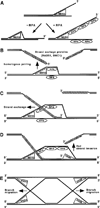
Fig. 7. Roles for MER3 helicase in DNA recombination
A, DNA unwinding reaction carried out by MER3 helicase with or without RPA. The polarity of MER3 helicase is in the 3′ to 5′ direction with respect to a single-stranded region. In the absence of single-stranded DNA-binding protein (−RPA), the region unwound by MER3 will re-anneal to form double-stranded DNA. In the presence of RPA (+RPA), the single-stranded regions produced by MER3 will be bound by RPA. RPA binding to the single-stranded regions prevents them from re-annealing to form double-stranded DNA. Thus, RPA stimulates the unwinding and subsequent displacement of long DNA fragments by MER3 helicase. There are several steps of DNA recombination at which the MER3 helicase might function. B, a possible role for MER3 in homologous pairing by unwinding double-stranded DNA prior to the initiation of DNA strand exchange. At each DSB site, two single-stranded DNA ends having 3′ -overhangs are formed. To create double-Holliday junctions that potentially result in crossovers, both of these ends must invade the same chromatid of homolog. C, once strand exchange has been initiated by RAD51 and/or DMC1, MER3 helicase could bind to the single-stranded region of the resulting D-loop and further unwind the duplex region with the aid of RPA. This would stimulate strand exchange during the first strand invasion. D, MER3 could play a similar role during the second strand invasion by unwinding the duplex region in the opposite direction as illustrated. This would stimulate strand exchange during the second strand invasion. E, it is also possible that MER3 helicase participates in branch migration of Holliday junctions following the first and second strand invasion.
Similar articles
- Saccharomyces cerevisiae Mer3 is a DNA helicase involved in meiotic crossing over.
Nakagawa T, Kolodner RD. Nakagawa T, et al. Mol Cell Biol. 2002 May;22(10):3281-91. doi: 10.1128/MCB.22.10.3281-3291.2002. Mol Cell Biol. 2002. PMID: 11971962 Free PMC article. - The Saccharomyces cerevisiae MER3 gene, encoding a novel helicase-like protein, is required for crossover control in meiosis.
Nakagawa T, Ogawa H. Nakagawa T, et al. EMBO J. 1999 Oct 15;18(20):5714-23. doi: 10.1093/emboj/18.20.5714. EMBO J. 1999. PMID: 10523314 Free PMC article. - Saccharomyces cerevisiae Mer3 helicase stimulates 3'-5' heteroduplex extension by Rad51; implications for crossover control in meiotic recombination.
Mazina OM, Mazin AV, Nakagawa T, Kolodner RD, Kowalczykowski SC. Mazina OM, et al. Cell. 2004 Apr 2;117(1):47-56. doi: 10.1016/s0092-8674(04)00294-6. Cell. 2004. PMID: 15066281 - Meiotic recombination: sealing the partnership at the junction.
Kunz C, Schär P. Kunz C, et al. Curr Biol. 2004 Nov 23;14(22):R962-4. doi: 10.1016/j.cub.2004.10.043. Curr Biol. 2004. PMID: 15556855 Review. - The Mus81 solution to resolution: generating meiotic crossovers without Holliday junctions.
Hollingsworth NM, Brill SJ. Hollingsworth NM, et al. Genes Dev. 2004 Jan 15;18(2):117-25. doi: 10.1101/gad.1165904. Genes Dev. 2004. PMID: 14752007 Free PMC article. Review. No abstract available.
Cited by
- HEIP1 regulates crossover formation during meiosis in rice.
Li Y, Qin B, Shen Y, Zhang F, Liu C, You H, Du G, Tang D, Cheng Z. Li Y, et al. Proc Natl Acad Sci U S A. 2018 Oct 16;115(42):10810-10815. doi: 10.1073/pnas.1807871115. Epub 2018 Oct 1. Proc Natl Acad Sci U S A. 2018. PMID: 30275327 Free PMC article. - A genetic screen for increased loss of heterozygosity in Saccharomyces cerevisiae.
Andersen MP, Nelson ZW, Hetrick ED, Gottschling DE. Andersen MP, et al. Genetics. 2008 Jul;179(3):1179-95. doi: 10.1534/genetics.108.089250. Epub 2008 Jun 18. Genetics. 2008. PMID: 18562670 Free PMC article. - Activation of Saccharomyces cerevisiae Mlh1-Pms1 Endonuclease in a Reconstituted Mismatch Repair System.
Smith CE, Bowen N, Graham WJ 5th, Goellner EM, Srivatsan A, Kolodner RD. Smith CE, et al. J Biol Chem. 2015 Aug 28;290(35):21580-90. doi: 10.1074/jbc.M115.662189. Epub 2015 Jul 13. J Biol Chem. 2015. PMID: 26170454 Free PMC article. - The Msh5 complex shows homeostatic localization in response to DNA double-strand breaks in yeast meiosis.
Shinohara M, Shinohara A. Shinohara M, et al. Front Cell Dev Biol. 2023 May 18;11:1170689. doi: 10.3389/fcell.2023.1170689. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37274743 Free PMC article. - The central element protein ZEP1 of the synaptonemal complex regulates the number of crossovers during meiosis in rice.
Wang M, Wang K, Tang D, Wei C, Li M, Shen Y, Chi Z, Gu M, Cheng Z. Wang M, et al. Plant Cell. 2010 Feb;22(2):417-30. doi: 10.1105/tpc.109.070789. Epub 2010 Feb 12. Plant Cell. 2010. PMID: 20154151 Free PMC article.
References
- Roeder GS. Genes Dev. 1997;11:2600–2621. - PubMed
- Sym M, Roeder GS. Cell. 1994;79:283–292. - PubMed
- Ross-Macdonald P, Roeder GS. Cell. 1994;79:1069–1080. - PubMed
- Hollingsworth NM, Ponte L, Halsey C. Genes Dev. 1995;9:1728–1739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous