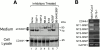Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration - PubMed (original) (raw)
Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration
M Kajita et al. J Cell Biol. 2001.
Abstract
Migratory cells including invasive tumor cells frequently express CD44, a major receptor for hyaluronan and membrane-type 1 matrix metalloproteinase (MT1-MMP) that degrades extracellular matrix at the pericellular region. In this study, we demonstrate that MT1-MMP acts as a processing enzyme for CD44H, releasing it into the medium as a soluble 70-kD fragment. Furthermore, this processing event stimulates cell motility; however, expression of either CD44H or MT1-MMP alone did not stimulate cell motility. Coexpression of MT1-MMP and mutant CD44H lacking the MT1-MMP-processing site did not result in shedding and did not promote cell migration, suggesting that the processing of CD44H by MT1-MMP is critical in the migratory stimulation. Moreover, expression of the mutant CD44H inhibited the cell migration promoted by CD44H and MT1-MMP in a dominant-negative manner. The pancreatic tumor cell line, MIA PaCa-2, was found to shed the 70-kD CD44H fragment in a MT1-MMP-dependent manner. Expression of the mutant CD44H in the cells as well as MMP inhibitor treatment effectively inhibited the migration, suggesting that MIA PaCa-2 cells indeed use the CD44H and MT1-MMP as migratory devices. These findings revealed a novel interaction of the two molecules that have each been implicated in tumor cell migration and invasion.
Figures
Figure 1
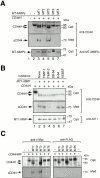
Shedding of CD44H by MT-MMPs. (A) CD44H was coexpressed with each of the MT-MMPs, as indicated by transient transfection of the expression plasmids into ZR-75-1 cells, and incubated in the serum-free media. After 48 h, cell lysates and medium fractions were collected and subjected to Western Blot analyses using monoclonal anti-CD44 and specific antibodies against each MT-MMP. (B) ZR-75-1 cells were transiently transfected with the expression plasmids for CD44H and MT1-MMP and cultured in serum-free media in the presence or absence of various proteinase inhibitors as indicated. After 48 h, cell lysates and medium fractions were collected and subjected to Western blot analyses. (C) CD44H with NH2-terminal c-Myc tag and COOH-terminal FLAG tag was coexpressed with each of the MT-MMPs, as indicated by transient transfection of the expression plasmids into ZR-75-1 cells, and analyzed the same as in A. The antibody against FLAG and c-Myc were used to determine the integrity of the peptide core of CD44H for the Western blot as indicated.
Figure 3
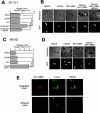
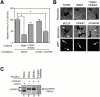
Effect of the shedding of CD44H by MT1-MMP on the cell motility. (A) ZR-75-1 cells were transfected with the expression plasmids for CD44H and/or MT1-MMP together with the one for GFP. The motility of GFP-positive cells was analyzed by phagokinetic track assay on colloidal gold–coated coverslips. The migrated area of the cell was visualized under darkfield illumination, and migration area was measured using NIH Image. The average of 30 cells ± SEM is shown. (B) Representative phagokinetic track of the migrating cell was visualized under darkfield illumination (Refraction). Transfected cells were indicated as GFP-positive cells (GFP). (C) Osteosarcoma MG-63 cells were analyzed as above. (D) Representative phagokinetic track of the migrating cell was visualized under darkfield illumination (Refraction). Transfected cells were indicated as GFP-positive cells (GFP). (E) MT1-MMP and CD44H expressing ZR-75-1 cells on glass coverslip were immunostained for CD44H and MT1-MMP. The signal was analyzed by confocal microscope. The combined image from all sections (top, Combined Sections) and one section from the cell attachment site is shown (bottom, Adherent Section). *P < 0.05 by Student's t test.
Figure 4
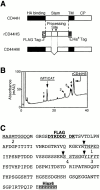
Processing of CD44H by MT1-MMP in vitro. (A) Schematic illustration of CD44H, the stem fragment (rCD44HS) expressed in E. coli, and the mutant lacking the processing sites by MT1-MMP (CD44HM). Fragments expressed in E. coli were tagged with FLAG at the NH2 terminus and His6 at the COOH terminus, as indicated. Processing sites (C) are indicated by the arrowheads. (B) rCD44HS (96 μg) was incubated with 3.6 μg of the catalytic fragment of MT1-MMP (rMT1CD) at 37°C for 180 min. Reaction products were separated by reverse-phase chromatography. Peaks indicated (peaks 1–4) were collected and subjected to automatic amino acid sequencer (Beckman Coulter LF3000). (C) Amino acid sequence of rCD44HS is presented. Thick letters at the NH2 terminus are the FLAG tag, and those at the COOH terminus are the His6 tag. The sequence between the tags corresponds to T130-E268 of CD44H. The determined NH2-terminal sequences of the peaks are underlined. Stem, the region between the HA-binding globular domain and transmembrane domain; TM, transmembrane domain; CP, cytoplasmic tail.
Figure 2
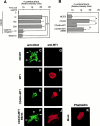
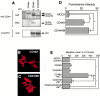
Effect of CD44 shedding on HA-binding activity and cell morphology. (A) ZR-75-1 cells were transfected with expression plasmid for CD44. After 24 h, the cells were incubated with increasing concentrations of FITC-labeled HA for 60 min at 37°C. After washing unbound FITC-HA, the relative intensity of green fluorescence of the transfected cells was analyzed by confocal laser microscopy. The average value of 40 individual cells was plotted (mean ± SEM). A 100-fold excess amount of cold-HA was used to compete FITC-HA binding. (B) ZR-75-1 cells were transfected with expression plasmids for CD44 and/or MT1-MMP. FITC-HA binding was analyzed similarly. ZR-75-1 cells were transfected with control vector (Mock), CD44H cDNA (CD44H), CD44H and MT1-MMP cDNAs (CD44H+MT1), CD44H and MT1-MMP cDNAs cultured in the presence of BB94 (CD44H+MT1/BB94). (C–K) ZR-75-1 cells transfected with expression plasmids indicated were cultured on glass slides. The cells were stained with rat anti–human CD44 and mouse anti–human MT1-MMP mAbs without permeabilization. Signals were visualized by further probing with Alexa 488–conjugated anti–rat IgG or Cy3-conjugated anti–mouse IgG and analyzed by a confocal laser microscope. Representative pictures are presented. Cells express CD44H (C and G), MT1-MMP (D and H), CD44H and MT1-MMP (E and I), CD44H and MT1-MMP cultured in the presence of BB94 (F and J), and mock-transfected cells (K). Cells were stained with ant-CD44 mAb (C–F) or anti–MT1-MMP (G–J). Mock cells were stained for F-actin by Cy3-conjugated phalloidin (K). *P < 0.05 by Student's t test.
Figure 6
Shedding of endogenous CD44H in human pancreatic tumor cell line, MIA PaCa-2. (A) MIA PaCa-2 cells (3 × 105) were cultured in a six-well plate in serum-free medium in the presence or absence of the proteinase inhibitors as indicated. After 48 h, the cell lysate (bottom) and the conditioned medium (top) were subjected to Western blot analyses. CD44 was detected by the mouse anti–human CD44 mAb. Concentrations of the inhibitors were adjusted as follows: 50 nM for TIMP-1, 50 nM for TIMP-2, 10 μM for BB94, 1.0 μM for E-64, and 1.0 mM for AEBSF. (B) Expression of genes for CD44 and MT-MMPs were examined by RT-PCR using specific primers as described in Materials and Methods. Sizes of the amplified fragments were 461 bp for CD44, 589 bp for MT1-MMP, 578 bp for MT2-MMP, 461 bp for MT3-MMP, 334 bp for MT4-MMP, 564 bp for MT5-MMP, and 500 bp for GAPDH. PCR product of CD44 indicates that CD44 expressed in MIA PaCa-2 is CD44H.
Figure 5
Dominant-negative effect of CD44HM on cell migration stimulated by CD44H and MT1-MMP. (A) Either wild-type CD44H or the mutant CD44HM was expressed in ZR-75-1 cells together with MT1-MMP. Cell lysate and medium fractions were subjected to Western blot analyses as described in the legend to Fig. 2. (B and C) CD44H- or CD44HM-expressing ZR-75-1 cells were stained with rat anti–human CD44 mAb and analyzed by confocal microscopy. (D) HA-binding activity of CD44H- or CD44HM-expressing ZR-75-1 were examined as described in the legend to Fig. 2. Transfected cells were incubated with FITC-HA for 3 h at 37°C, and fluorescence associated to the cells was measured. (E) Transfected ZR-75-1 cells were subjected to the migration assay as described in the legend to Fig. 3. *P < 0.05 by Student's t test.
Figure 7
BB94 and CD44HM inhibits motile activity of MIA PaCa-2. (A) MIA PaCa-2 cells were transfected with the expression plasmid for the FLAG-tagged CD44H or CD44HM together with the one for GFP. After 48 h, cells were subjected to the migration assay as described in the legend to Fig. 4. Mock- or CD44HM-transfected cells were cultured in the presence or absence of BB94 or trypsin inhibitor. (B) Representative phagokinetic track of the migrating cell was visualized under darkfield illumination (Refraction). Transfected cells were indicated as GFP-positive cells (GFP; arrow). (C) Medium fractions from same transfectants were subjected to Western blot analyses using anti-FLAG M2 mAb as described in the legend to Fig. 1. *P < 0.05 by Student's t test.
Similar articles
- CD44 binding through the hemopexin-like domain is critical for its shedding by membrane-type 1 matrix metalloproteinase.
Suenaga N, Mori H, Itoh Y, Seiki M. Suenaga N, et al. Oncogene. 2005 Jan 27;24(5):859-68. doi: 10.1038/sj.onc.1208258. Oncogene. 2005. PMID: 15558018 - CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain.
Mori H, Tomari T, Koshikawa N, Kajita M, Itoh Y, Sato H, Tojo H, Yana I, Seiki M. Mori H, et al. EMBO J. 2002 Aug 1;21(15):3949-59. doi: 10.1093/emboj/cdf411. EMBO J. 2002. PMID: 12145196 Free PMC article. - Constitutive and induced CD44 shedding by ADAM-like proteases and membrane-type 1 matrix metalloproteinase.
Nakamura H, Suenaga N, Taniwaki K, Matsuki H, Yonezawa K, Fujii M, Okada Y, Seiki M. Nakamura H, et al. Cancer Res. 2004 Feb 1;64(3):876-82. doi: 10.1158/0008-5472.can-03-3502. Cancer Res. 2004. PMID: 14871815 - Role of pericellular proteolysis by membrane-type 1 matrix metalloproteinase in cancer invasion and angiogenesis.
Seiki M, Koshikawa N, Yana I. Seiki M, et al. Cancer Metastasis Rev. 2003 Jun-Sep;22(2-3):129-43. doi: 10.1023/a:1023087113214. Cancer Metastasis Rev. 2003. PMID: 12784992 Review. - Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion.
Seiki M. Seiki M. Cancer Lett. 2003 May 8;194(1):1-11. doi: 10.1016/s0304-3835(02)00699-7. Cancer Lett. 2003. PMID: 12706853 Review.
Cited by
- Constitutive and Regulated Shedding of Soluble FGF Receptors Releases Biologically Active Inhibitors of FGF-2.
Hanneken A, Mercado M, Maher P. Hanneken A, et al. Int J Mol Sci. 2021 Mar 8;22(5):2712. doi: 10.3390/ijms22052712. Int J Mol Sci. 2021. PMID: 33800200 Free PMC article. - Chemotherapy induces feedback up-regulation of CD44v6 in colorectal cancer initiating cells through _β_-catenin/MDR1 signaling to sustain chemoresistance.
Ghatak S, Hascall VC, Karamanos N, Markwald RR, Misra S. Ghatak S, et al. Front Oncol. 2022 Oct 18;12:906260. doi: 10.3389/fonc.2022.906260. eCollection 2022. Front Oncol. 2022. PMID: 36330477 Free PMC article. - The overexpression membrane type 1 matrix metalloproteinase is associated with the progression and prognosis in breast cancer.
Li Y, Cai G, Yuan S, Jun Y, Li N, Wang L, Chen F, Ling R, Yun J. Li Y, et al. Am J Transl Res. 2015 Jan 15;7(1):120-7. eCollection 2015. Am J Transl Res. 2015. PMID: 25755834 Free PMC article. - Combined analysis of single-cell sequencing and bulk transcriptome sequencing reveals new mechanisms for non-healing diabetic foot ulcers.
Chen R, Zou L. Chen R, et al. PLoS One. 2024 Jul 1;19(7):e0306248. doi: 10.1371/journal.pone.0306248. eCollection 2024. PLoS One. 2024. PMID: 38950058 Free PMC article. - Interplay between Cell-Surface Receptors and Extracellular Matrix in Skin.
Kleiser S, Nyström A. Kleiser S, et al. Biomolecules. 2020 Aug 11;10(8):1170. doi: 10.3390/biom10081170. Biomolecules. 2020. PMID: 32796709 Free PMC article. Review.
References
- Albrecht-Buehler G. The phagokinetic tracks of 3T3 cells. Cell. 1977;11:395–404. - PubMed
- Bartolazzi A., Jackson D., Bennett K., Aruffo A., Dickinson R., Shields J., Whittle N., Stamenkovic I. Regulation of growth and dissemination of a human lymphoma by CD44 splice variants. J. Cell Sci. 1995;108:1723–1733. - PubMed
- Basbaum C.B., Werb Z. Focalized proteolysisspatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr. Opin. Cell Biol. 1996;8:731–738. - PubMed
- Bazil V., Strominger J.L. Metalloprotease and serine protease are involved in cleavage of CD43, CD44, and CD16 from stimulated human granulocytes. Induction of cleavage of L-selectin via CD16. J. Immunol. 1994;152:1314–1322. - PubMed
- Borland G., Murphy G., Ager A. Tissue inhibitor of metalloproteinases-3 inhibits shedding of L- selectin from leukocytes. J. Biol. Chem. 1999;274:2810–2815. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous