E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner - PubMed (original) (raw)
E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner
C J Gottardi et al. J Cell Biol. 2001.
Abstract
E-cadherin is a tumor suppressor protein with a well-established role in cell-cell adhesion. Adhesion could contribute to tumor suppression either by physically joining cells or by facilitating other juxtacrine signaling events. Alternatively, E-cadherin tumor suppressor activity could result from binding and antagonizing the nuclear signaling function of beta-catenin, a known proto-oncogene. To distinguish between an adhesion- versus a beta-catenin signaling-dependent mechanism, chimeric cadherin constructs were expressed in the SW480 colorectal tumor cell line. Expression of wild-type E-cadherin significantly inhibits the growth of this cell line. Growth inhibitory activity is retained by all constructs that have the beta-catenin binding region of the cytoplasmic domain but not by E-cadherin constructs that exhibit adhesive activity, but lack the beta-catenin binding region. This growth suppression correlates with a reduction in beta-catenin/T cell factor (TCF) reporter gene activity. Importantly, direct inhibition of beta-catenin/TCF signaling inhibits the growth of SW480 cells, and the growth inhibitory activity of E-cadherin is rescued by constitutively activated forms of TCF. Thus, the growth suppressor activity of E-cadherin is adhesion independent and results from an inhibition of the beta-catenin/TCF signaling pathway, suggesting that loss of E-cadherin expression can contribute to upregulation of this pathway in human cancers. E-cadherin-mediated growth suppression was not accompanied by overall depletion of beta-catenin from the cytosol and nucleus. This appears to be due to the existence of a large pool of cytosolic beta-catenin in SW480 cells that is refractory to both cadherin binding and TCF binding. Thus, a small pool of beta-catenin that can bind TCF (i.e., the transcriptionally active pool) can be selectively depleted by E-cadherin expression. The existence of functionally distinct pools of cytosolic beta-catenin suggests that there are mechanisms to regulate beta-catenin signaling in addition to controlling its level of accumulation.
Figures
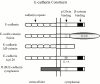
Figure 1
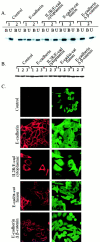
Schematic diagram of E-cadherin constructs used in this study. Wild-type E-cadherin (top) is shown. The next two constructs (middle) were designed to mediate adhesion without interacting with β-catenin: E-cadherin–α-catenin fusion construct joins the extracellular and membrane proximal (p120ctn binding) region of E-cadherin directly with α-catenin; the E-cadherin Δ β-catenin construct is truncated before the β-catenin binding region. The E-cadherin Δ p120ctn construct contains 3 Gly > Ala point mutations and therefore cannot interact with p120ctn. The IL2R/E-cadherin cytoplasmic chimera fuses the extracellular and transmembrane domains of the interleukin-2 receptor α subunit to the cytoplasmic domain of E-cadherin. This construct can bind β-catenin but cannot engage in homophilic adhesive activity.
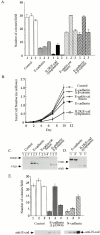
Figure 2
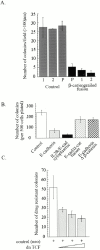
Growth properties of cadherin construct–expressing cell lines. Three independent clones per construct were analyzed. (A) Anchorage-independent growth. An equivalent number of cells from each stable cell line was seeded into soft agar, and colonies >100 μm diameter were counted after ∼14 d in culture. Three independent clones per construct (columns 1, 2, and 3) were characterized (± SEM). (B) Growth properties of cell clones on plastic. Each data point represents the mean from the three independent cell lines. (C and D) Western blot analysis and relative expression levels of cadherin construct–expressing cell lines after immunoblotting with an mAb to the extracellular domain of E-cadherin (HECD-1) (C), or with an antibody that recognizes the cytoplasmic domain of the cadherin (D) (Pep1 polyclonal antibody; Choi and Gumbiner 1989). (E) Anchorage-independent growth properties and Western analysis of E-cadherin Δ p120ctn and N-cadherin. Mock-transfected (Control), E-cadherin Δ p120ctn, and N-cadherin–expressing cell lines were seeded into soft agar, and the number of colonies was counted after ∼15 d. Western analysis of E-cadherin Δ p120ctn and N-cadherin–expressing cell lines is shown.
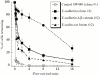
Figure 3
Adhesive properties of cadherin-expressing cell lines using a laminar flow cell attachment assay. Mock-transfected control SW480 cells and SW480 cells expressed wild-type E-cadherin, E-cadherin Δ β-catenin, or an E-cadherin–α-catenin fusion, remaining attached to glass capillary tubes coated with the extracellular domain of human E-cadherin after increasing flow rates (ml/min). Each curve represents adhesive activity of a given construct-expressing cell line. Adhesive activity shown above was completely calcium dependent (data not shown).
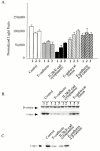
Figure 4
Activities of β-catenin/TCF target genes. E-cadherin or E-cadherin construct–expressing SW480 cell lines. (A) β-catenin/TCF-dependent reporter gene assay. Individual cell lines were transiently transfected with the β-catenin/TCF–dependent luciferase reporter gene (TOPFLASH) and a β-galactosidase reporter plasmid to control for transfection efficiency. Negligible activity was observed when the TCF binding sites were mutated (FOPFLASH; not shown). (B and C) Expression of product of endogenous β-catenin/TCF target gene, c-myc: control and cadherin construct–expressing cell lines were analyzed by SDS-PAGE and immunoblotting with β-catenin and c-myc specific antibodies. Individual cell lines were examined in B; cell lines were pooled in C.
Figure 5
Inhibition of β-catenin/TCF gene activation sup-presses SW480 cell growth. (A) Anchorage-independent growth of β-catenin/engrailed repressor–expressing cell lines. (B) Colony-forming assay reflects the growth properties of stably transfected cadherin construct–expressing cell lines. The stable cell lines characterized for growth in soft agar and growth on plastic in Fig. 2 were assessed for their ability to inhibit colony formation when plated under dilute conditions (500 cells plated per dish). Each bar represents the mean of three independent cell lines evaluated for each cadherin construct. (C) Dominant-negative TCF inhibits growth of SW480 cells, as assessed with the colony-forming assay. Cells were transfected with a neomycin drug resistance plasmid (control [neo] +) with or without increasing amounts (0.002, 0.02, and 0.2 μg) of plasmid expressing a dominant negative (dn TCF). Results are from triplicate transfections (±SEM).
Figure 6
Constitutively activated forms of LEF and TCF rescue cadherin-mediated growth inhibition of SW480 cells. (A) Colony formation assay after transfection with E-cadherin or E-cadherin plus activated LEF (β-catenin COOH-terminal transactivation domain fused directly to LEF). Control plasmid (pcDNA3neo), E-cadherin/pcDNA3neo plasmid (E-cadherin [neo]) or activated LEF (0.0001–1.0 μg). (B) Colony–forming assay after transfection with E-cadherin or E-cadherin plus activated TCF (VP16 transactivation domain fused to TCF-3), and wild-type TCF (wtTCF) or dominant-negative TCF (dnTCF) as controls. TCF-encoding plasmids were titrated for optimal effect (0.001–0.1 μg). Bars represent mean number of colonies from triplicate transfections (±SEM).
Figure 8
SW480 cells contain a large pool of β-catenin that does not interact with either cadherins or TCF. (A) Only a small fraction of cytosolic β–catenin is competent to interact with the cadherin cytoplasmic domain in vitro. Sequential depletion of β-catenin (1st, 2nd, and 3rd) from a detergent-free 100,000-g supernatant fraction of SW480 cells with cadherin–GST (cad-GST), and TCA precipitation of the unbound fraction. (B) Only a small fraction of cytosolic β-catenin is competent to interact with the TCF in vitro. Sequential depletion of β-catenin from the 100,000-g supernatant fraction of SW480 cells with TCF–GST, and TCA precipitation of the unbound fraction. (C) Cadherin-unbindable pool of β-catenin does not interact with TCF. Sequential depletion of β-catenin from an SW480 cell detergent lysate with cad-GST (1st, 2nd, and 3rd), and final depleted fraction subject to either TCA precipitation (unbound) or binding to TCF–GST. (D) TCF-unbindable pool of β-catenin does not interact with the cadherin cytoplasmic domain. Sequential depletion of β-catenin from an SW480 cell detergent lysate with TCF–GST (1st, 2nd, 3rd), and final depleted fraction subject to either TCA precipitation (unbound) or binding to cad-GST. Samples were separated by SDS-PAGE, and β-catenin was detected by immunoblotting.
Figure 7
Levels and subcellular distribution of β-catenin are not significantly altered in cadherin construct–expressing SW480 cell lines. (A) Fractionation of β-catenin into cadherin-associated and soluble pools using the lectin ConA. Detergent lysates were incubated with ConA-Sepharose (amount predetermined to deplete all of the cadherin) and equivalent proportions of the ConA-bound (B) and -unbound (U) fractions were analyzed by SDS-PAGE and immunoblotting. Two cell lines per constructs were analyzed. (B) Total levels of β-catenin: Western blot of SDS lysates of individual cell lines. (C) Indirect immunofluorescence microscopy of cadherin-constructs (CY-3) and β-catenin (FITC) in control and cadherin construct–expressing stable cell lines.
Similar articles
- Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin.
Wong AS, Gumbiner BM. Wong AS, et al. J Cell Biol. 2003 Jun 23;161(6):1191-203. doi: 10.1083/jcb.200212033. Epub 2003 Jun 16. J Cell Biol. 2003. PMID: 12810698 Free PMC article. - Role for ICAT in beta-catenin-dependent nuclear signaling and cadherin functions.
Gottardi CJ, Gumbiner BM. Gottardi CJ, et al. Am J Physiol Cell Physiol. 2004 Apr;286(4):C747-56. doi: 10.1152/ajpcell.00433.2003. Epub 2003 Nov 12. Am J Physiol Cell Physiol. 2004. PMID: 14613891 - Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes.
Gottardi CJ, Gumbiner BM. Gottardi CJ, et al. J Cell Biol. 2004 Oct 25;167(2):339-49. doi: 10.1083/jcb.200402153. Epub 2004 Oct 18. J Cell Biol. 2004. PMID: 15492040 Free PMC article. - Signaling through beta-catenin and Lef/Tcf.
Novak A, Dedhar S. Novak A, et al. Cell Mol Life Sci. 1999 Oct 30;56(5-6):523-37. doi: 10.1007/s000180050449. Cell Mol Life Sci. 1999. PMID: 11212302 Free PMC article. Review. - A possible role for the WNT-1 pathway in oral carcinogenesis.
Lo Muzio L. Lo Muzio L. Crit Rev Oral Biol Med. 2001;12(2):152-65. doi: 10.1177/10454411010120020501. Crit Rev Oral Biol Med. 2001. PMID: 11345525 Review.
Cited by
- ULK1 Mediated Autophagy-Promoting Effects of Rutin-Loaded Chitosan Nanoparticles Contribute to the Activation of NF-κB Signaling Besides Inhibiting EMT in Hep3B Hepatoma Cells.
Wu P, Wang X, Yin M, Zhu W, Chen Z, Zhang Y, Jiang Z, Shi L, Zhu Q. Wu P, et al. Int J Nanomedicine. 2024 May 18;19:4465-4493. doi: 10.2147/IJN.S443117. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38779103 Free PMC article. - Novel chromium (III)-based compound for inhibition of oxaliplatin-resistant colorectal cancer progression.
Chen MC, Devi HS, Pien HF, Wen SF, Sheu JL, Tsai BC, Huang CY, Lin YJ. Chen MC, et al. Am J Cancer Res. 2024 Mar 15;14(3):979-995. doi: 10.62347/XTRT2780. eCollection 2024. Am J Cancer Res. 2024. PMID: 38590406 Free PMC article. - Dynamic interplay of nuclear receptors in tumor cell plasticity and drug resistance: Shifting gears in malignant transformations and applications in cancer therapeutics.
BharathwajChetty B, Sajeev A, Vishwa R, Aswani BS, Alqahtani MS, Abbas M, Kunnumakkara AB. BharathwajChetty B, et al. Cancer Metastasis Rev. 2024 Mar;43(1):321-362. doi: 10.1007/s10555-024-10171-0. Epub 2024 Mar 22. Cancer Metastasis Rev. 2024. PMID: 38517618 Review. - Research progress on the regulatory mechanism of integrin-mediated mechanical stress in cells involved in bone metabolism.
Yang L, Chen H, Yang C, Hu Z, Jiang Z, Meng S, Liu R, Huang L, Yang K. Yang L, et al. J Cell Mol Med. 2024 Apr;28(7):e18183. doi: 10.1111/jcmm.18183. J Cell Mol Med. 2024. PMID: 38506078 Free PMC article. Review. - Endolysosomal TRPML1 channel regulates cancer cell migration by altering intracellular trafficking of E-cadherin and β1-integrin.
Frey N, Ouologuem L, Blenninger J, Siow WX, Thorn-Seshold J, Stöckl J, Abrahamian C, Fröhlich T, Vollmar AM, Grimm C, Bartel K. Frey N, et al. J Biol Chem. 2024 Jan;300(1):105581. doi: 10.1016/j.jbc.2023.105581. Epub 2023 Dec 21. J Biol Chem. 2024. PMID: 38141765 Free PMC article.
References
- Ahmed Y., Hayashi S., Levine A., Wieschaus E. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell. 1998;93:1171–1182. - PubMed
- Behrens J., von Kries J.P., Kuhl M., Bruhn L., Wedlich D., Grosschedl R., Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
- Behrens J., Jerchow B.A., Wurtele M., Grimm J., Asbrand C., Wirtz R., Kuhl M., Wedlich D., Birchmeier W. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science. 1998;280:596–599. - PubMed
- Birchmeier W., Behrens J. Cadherin expression in carcinomasrole in the formation of cell junctions and the prevention of invasiveness. Biochim. Biophys. Acta. 1994;1198:11–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous