The yeast class V myosins, Myo2p and Myo4p, are nonprocessive actin-based motors - PubMed (original) (raw)
The yeast class V myosins, Myo2p and Myo4p, are nonprocessive actin-based motors
S L Reck-Peterson et al. J Cell Biol. 2001.
Erratum in
- J Cell Biol 2001 Jun 25;153(7):1521
Abstract
The motor properties of the two yeast class V myosins, Myo2p and Myo4p, were examined using in vitro motility assays. Both myosins are active motors with maximum velocities of 4.5 microm/s for Myo2p and 1.1 microm/s for Myo4p. Myo2p motility is Ca(2+) insensitive. Both myosins have properties of a nonprocessive motor, unlike chick myosin-Va (M5a), which behaves as a processive motor when assayed under identical conditions. Additional support for the idea that Myo2p is a nonprocessive motor comes from actin cosedimentation assays, which show that Myo2p has a low affinity for F-actin in the presence of ATP and Ca(2+), unlike chick brain M5a. These studies suggest that if Myo2p functions in organelle transport, at least five molecules of Myo2p must be present per organelle to promote directed movement.
Figures
Figure 2
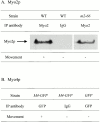
Motility of Myo2p, Myo2-66p, and Myo4p. (A) Immunoblot analysis of antibody capture motility chambers containing either anti Myo-2p or nonimmune IgG. Chambers were incubated with S3 supernates from either wild-type (WT) or myo2-66 cells. Only chambers containing anti-Myo2p and wild-type S3 exhibited movement, although the myo2-66p was present in the chamber. No Myo2p was present in the IgG control chamber. (B) Summary of motility results using anti-GFP to adsorb Myo4-GFPp. Immunoadsorbtions were performed from both tagged and untagged strains. Movement was observed only in chambers containing anti-GFP and S3 supernate from Myo4-GFPp–expressing cells.
Figure 1
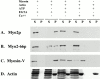
Actin cosedimentation assays. Myo2p (A), Myo2-66p (B), or chick brain M5a (C) in S3 supernates was incubated with F-actin in the presence or absence of 4.0 mM ATP with or without 50 μM free Ca2+. After centrifugation to pellet, the F-actin, the supernate, and pellet fractions were immunoblotted with antibodies to Myo2p (A and B) or M5a (C). D shows a representative Coomassie blue–stained gel to visualize actin. In contrast to M5a, Myo2p fails to bind to actin in the presence of ATP. The motor mutant Myo2-66p exhibits reduced actin binding activity.
Figure 3
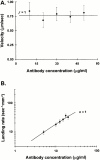
Chick brain M5a is a processive motor when assayed using the antibody capture (from S3) in vitro motility assay. (A) Velocity (μm/s ± SD) as a function of M5a tail antibody concentration. No movement was observed at an antibody concentration of 5 μg/ ml. The solid line is a theoretical curve derived from with the duty ratio, f = 1 (see Materials and Methods). The good fit to this line indicates that a single M5a molecule remains strongly bound to an actin filament for nearly its entire catalytic cycle. (B) Landing rate (n ± standard error, where n is the number of landing events per unit area, per unit time; see Materials and Methods) as a function of antibody concentration. The curve fit, derived from , represents the case where n = 1, i.e., only one motor molecule is required to bind a filament and initiate movement.
Figure 4
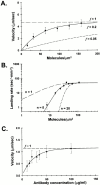
Myo2p and Myo4p are nonprocessive class V myosins. (A) Average velocity (μm/s ± SD) is plotted as a function of motor concentration. No movement was seen at a Myo2p concentration of 8.0 molecules/μm2. Myo2p motility data were best fit by the theoretical curve representing a duty cycle, f = 0.2 (solid line), indicating that a Myo2p molecule is only strongly bound to actin for ∼ 20% of its entire catalytic cycle. Also shown are theoretical curves representing duty ratios of 1.0 (similar to chick brain M5a, long dash) and 0.05 (similar to myosin-II, short dash). (B) Landing rate (n ± standard error, where n is the number of landing events per unit area, per unit time) is plotted as a function of motor concentration. The landing rate was best fit with Eq.4 when n = 5 (solid line; see Materials and Methods), indicating that at least five Myo2p molecules are needed to bind and initiate the movement of an actin filament. Also shown are theoretical landing rate fits where n is equal to 1 (a processive motor such as M5a, long dash) or 20 (a highly nonprocessive motor, short dash). (C) Myo4p velocity (μm/s ± SD) as a function of anti-GFP antibody concentration. No movement was observed using an antibody concentration of 5 μg/ml.
Similar articles
- Myo4p is a monomeric myosin with motility uniquely adapted to transport mRNA.
Dunn BD, Sakamoto T, Hong MS, Sellers JR, Takizawa PA. Dunn BD, et al. J Cell Biol. 2007 Sep 24;178(7):1193-206. doi: 10.1083/jcb.200707080. J Cell Biol. 2007. PMID: 17893244 Free PMC article. - Evidence for F-actin-dependent and -independent mechanisms involved in assembly and stability of the medial actomyosin ring in fission yeast.
Naqvi NI, Eng K, Gould KL, Balasubramanian MK. Naqvi NI, et al. EMBO J. 1999 Feb 15;18(4):854-62. doi: 10.1093/emboj/18.4.854. EMBO J. 1999. PMID: 10022828 Free PMC article. - Polarized distribution of intracellular components by class V myosins in Saccharomyces cerevisiae.
Matsui Y. Matsui Y. Int Rev Cytol. 2003;229:1-42. doi: 10.1016/s0074-7696(03)29001-x. Int Rev Cytol. 2003. PMID: 14669953 Review. - The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting.
Schott D, Ho J, Pruyne D, Bretscher A. Schott D, et al. J Cell Biol. 1999 Nov 15;147(4):791-808. doi: 10.1083/jcb.147.4.791. J Cell Biol. 1999. PMID: 10562281 Free PMC article. - Cytokinesis in fission yeast: a myosin pas de deux.
Mulvihill DP, Win TZ, Pack TP, Hyams JS. Mulvihill DP, et al. Microsc Res Tech. 2000 Apr 15;49(2):152-60. doi: 10.1002/(SICI)1097-0029(20000415)49:2<152::AID-JEMT7>3.0.CO;2-7. Microsc Res Tech. 2000. PMID: 10816254 Review.
Cited by
- Actomyosin Complex.
Pepper I, Galkin VE. Pepper I, et al. Subcell Biochem. 2022;99:421-470. doi: 10.1007/978-3-031-00793-4_14. Subcell Biochem. 2022. PMID: 36151385 Free PMC article. - Assembly of mRNA-protein complexes for directional mRNA transport in eukaryotes--an overview.
Jansen RP, Niessing D. Jansen RP, et al. Curr Protein Pept Sci. 2012 Jun;13(4):284-93. doi: 10.2174/138920312801619493. Curr Protein Pept Sci. 2012. PMID: 22708485 Free PMC article. Review. - Myo4p is a monomeric myosin with motility uniquely adapted to transport mRNA.
Dunn BD, Sakamoto T, Hong MS, Sellers JR, Takizawa PA. Dunn BD, et al. J Cell Biol. 2007 Sep 24;178(7):1193-206. doi: 10.1083/jcb.200707080. J Cell Biol. 2007. PMID: 17893244 Free PMC article. - Myosin Vc is a molecular motor that functions in secretory granule trafficking.
Jacobs DT, Weigert R, Grode KD, Donaldson JG, Cheney RE. Jacobs DT, et al. Mol Biol Cell. 2009 Nov;20(21):4471-88. doi: 10.1091/mbc.e08-08-0865. Epub 2009 Sep 9. Mol Biol Cell. 2009. PMID: 19741097 Free PMC article. - Myo4p and She3p are required for cortical ER inheritance in Saccharomyces cerevisiae.
Estrada P, Kim J, Coleman J, Walker L, Dunn B, Takizawa P, Novick P, Ferro-Novick S. Estrada P, et al. J Cell Biol. 2003 Dec 22;163(6):1255-66. doi: 10.1083/jcb.200304030. J Cell Biol. 2003. PMID: 14691136 Free PMC article.
References
- Cheney R.E. Purification and assay of myosin V. Methods Enzymol. 1998;298:3–18. - PubMed
- Cheney R.E., O'Shea M.K., Heuser J.E., Coelho M.V., Wolenski J.S., Espreafico E.M., Forscher P., Larson R.E., Mooseker M.S. Brain myosin-V is a two-headed unconventional myosin with motor activity Cell 75 1993. 13 23a - PubMed
- Cheney R.E., Riley M.A., Mooseker M.S. Phylogenetic analysis of the myosin superfamily Cell. Motil. Cytoskeleton 24 1993. 215 223b - PubMed
- Coluccio L.M., Geeves M.A. Transient kinetic analysis of the 130-kDa myosin I (MYR-1 gene product) from rat liver. A myosin I designed for maintenance of tension? J. Biol. Chem. 1999;274:21575–21580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 DK025387/DK/NIDDK NIH HHS/United States
- P01 CA046128/CA/NCI NIH HHS/United States
- DK 25387/DK/NIDDK NIH HHS/United States
- DK-10113/DK/NIDDK NIH HHS/United States
- F32 DK010113/DK/NIDDK NIH HHS/United States
- R37 DK025387/DK/NIDDK NIH HHS/United States
- P01 DK055389/DK/NIDDK NIH HHS/United States
- DK 55389/DK/NIDDK NIH HHS/United States
- R01 DK025387/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous