MAGI-1c: a synaptic MAGUK interacting with muSK at the vertebrate neuromuscular junction - PubMed (original) (raw)
MAGI-1c: a synaptic MAGUK interacting with muSK at the vertebrate neuromuscular junction
L Strochlic et al. J Cell Biol. 2001.
Abstract
The muscle-specific receptor tyrosine kinase (MuSK) forms part of a receptor complex, activated by nerve-derived agrin, that orchestrates the differentiation of the neuromuscular junction (NMJ). The molecular events linking MuSK activation with postsynaptic differentiation are not fully understood. In an attempt to identify partners and/or effectors of MuSK, cross-linking and immunopurification experiments were performed in purified postsynaptic membranes from the Torpedo electrocyte, a model system for the NMJ. Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) analysis was conducted on both cross-link products, and on the major peptide coimmunopurified with MuSK; this analysis identified a polypeptide corresponding to the COOH-terminal fragment of membrane-associated guanylate kinase (MAGUK) with inverted domain organization (MAGI)-1c. A bona fide MAGI-1c (150 kD) was detected by Western blotting in the postsynaptic membrane of Torpedo electrocytes, and in a high molecular mass cross-link product of MuSK. Immunofluorescence experiments showed that MAGI-1c is localized specifically at the adult rat NMJ, but is absent from agrin-induced acetylcholine receptor clusters in myotubes in vitro. In the central nervous system, MAGUKs play a primary role as scaffolding proteins that organize cytoskeletal signaling complexes at excitatory synapses. Our data suggest that a protein from the MAGUK family is involved in the MuSK signaling pathway at the vertebrate NMJ.
Figures
Figure 1
MuSK cross-link products and immunopurification of MuSK complexes. (a) Proteins from Torpedo AChR-rich membranes were cross-linked with various concentrations of SMPB (lane 0, control; lane 1, 7 × 10−6 M; lane 2, 2 × 10−5 M; lane 3, 4 × 10−5 M; lane 4, 10−4 M; lane 5, 2.5 × 10−4 M) and separated by SDS-PAGE. Western blots revealed that in addition to MuSK (97 kD), a 140-kD cross-link product was detected at low SMPB concentrations. Other cross-link products were observed at higher concentrations. (b) Purification of uncross-linked (−SMPB) and cross-linked (+SMPB, 4 × 10−5 M) MuSK complexes was achieved by immunoaffinity chromatography. The immunopurified polypeptides or the MuSK cross-link products were detected by silver staining (ST) or identified by immunoblotting (IB), respectively. In both experiments, a 40-kD polypeptide was detected in addition to MuSK. After SMPB treatment, a major cross-link product of 140 kD was detected. The asterisk indicates residual IgGs.
Figure 2
MALDI-TOF mass spectometry analysis of Torpedo MuSK binding partners. (a–c) Coverage maps of experimental tryptic peptides compared with theoretical tryptic peptides from databases obtained with ProFound™ software were shown. Coverages of 6% with rat MuSK (a), and of 16% with the COOH-terminal peptide (residues 1124–1374) of mouse MAGI-1c (b) were obtained from the 140-kD cross-link product. A coverage of 35% with the COOH-terminal peptide of MAGI-1c was obtained from the 40-kD polypeptide coimmunopurified with MuSK (c).
Figure 3
Characterization of the MuSK–MAGI-1c interaction in Torpedo postsynaptic membranes. (a) Western blots of AChR-rich Torpedo membranes were probed with preimmune serum (lane 1), and anti–MAGI-1c (R 85) (lane 2). A bona fide MAGI-1c (150 kD) and a 40-kD putative proteolytic fragment were detected (lane 2). (b) Western blots probed with R 497 and mab 2847 antibodies show that full-length MAGI-1 and MuSK were present together in a 250-kD cross-link product. (c) MuSK was coimmunopurified with the COOH-terminal fragment of MAGI-1c upon immunoaffinity chromatography with R 84 or R 85 antibodies. The immunopurified polypeptides were detected by silver staining (ST), and MuSK was detected by immunoblotting.
Figure 4
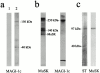
MAGI-1c localizes at the Torpedo and rat cholinergic synapses. (a) Double-fluorescence experiment showing the colocalisation of MAGI-1 (antibody R 499) and AChRs (α-bungarotoxin staining) at the innervated faces of Torpedo electrocytes. (b) In rat skeletal muscle fibers, MAGI-1 (antibody R 499) also strictly colocalized with AChRs at the NMJs. (c) At variance, MAGI-1 was not detected in agrin-induced AChR clusters in C2C12 myotubes. Bars, 30 μm.
Similar articles
- 14-3-3 gamma associates with muscle specific kinase and regulates synaptic gene transcription at vertebrate neuromuscular synapse.
Strochlic L, Cartaud A, Mejat A, Grailhe R, Schaeffer L, Changeux JP, Cartaud J. Strochlic L, et al. Proc Natl Acad Sci U S A. 2004 Dec 28;101(52):18189-94. doi: 10.1073/pnas.0406905102. Epub 2004 Dec 16. Proc Natl Acad Sci U S A. 2004. PMID: 15604144 Free PMC article. - Distinct domains of MuSK mediate its abilities to induce and to associate with postsynaptic specializations.
Zhou H, Glass DJ, Yancopoulos GD, Sanes JR. Zhou H, et al. J Cell Biol. 1999 Sep 6;146(5):1133-46. doi: 10.1083/jcb.146.5.1133. J Cell Biol. 1999. PMID: 10477765 Free PMC article. - The Agrin/MuSK signaling pathway is spatially segregated from the neuregulin/ErbB receptor signaling pathway at the neuromuscular junction.
Trinidad JC, Fischbach GD, Cohen JB. Trinidad JC, et al. J Neurosci. 2000 Dec 1;20(23):8762-70. doi: 10.1523/JNEUROSCI.20-23-08762.2000. J Neurosci. 2000. PMID: 11102484 Free PMC article. - The synaptic muscle-specific kinase (MuSK) complex: new partners, new functions.
Strochlic L, Cartaud A, Cartaud J. Strochlic L, et al. Bioessays. 2005 Nov;27(11):1129-35. doi: 10.1002/bies.20305. Bioessays. 2005. PMID: 16237673 Review. - Structure and activation of MuSK, a receptor tyrosine kinase central to neuromuscular junction formation.
Hubbard SR, Gnanasambandan K. Hubbard SR, et al. Biochim Biophys Acta. 2013 Oct;1834(10):2166-9. doi: 10.1016/j.bbapap.2013.02.034. Epub 2013 Mar 5. Biochim Biophys Acta. 2013. PMID: 23467009 Free PMC article. Review.
Cited by
- Mass spectrometry analysis of HIV-1 Vif reveals an increase in ordered structure upon oligomerization in regions necessary for viral infectivity.
Auclair JR, Green KM, Shandilya S, Evans JE, Somasundaran M, Schiffer CA. Auclair JR, et al. Proteins. 2007 Nov 1;69(2):270-84. doi: 10.1002/prot.21471. Proteins. 2007. PMID: 17598142 Free PMC article. - Fundamental Molecules and Mechanisms for Forming and Maintaining Neuromuscular Synapses.
Burden SJ, Huijbers MG, Remedio L. Burden SJ, et al. Int J Mol Sci. 2018 Feb 6;19(2):490. doi: 10.3390/ijms19020490. Int J Mol Sci. 2018. PMID: 29415504 Free PMC article. Review. - A single pulse of agrin triggers a pathway that acts to cluster acetylcholine receptors.
Mittaud P, Camilleri AA, Willmann R, Erb-Vögtli S, Burden SJ, Fuhrer C. Mittaud P, et al. Mol Cell Biol. 2004 Sep;24(18):7841-54. doi: 10.1128/MCB.24.18.7841-7854.2004. Mol Cell Biol. 2004. PMID: 15340048 Free PMC article. - Liprin-α-1 is a novel component of the murine neuromuscular junction and is involved in the organization of the postsynaptic machinery.
Bernadzki KM, Gawor M, Pęziński M, Mazurek P, Niewiadomski P, Rędowicz MJ, Prószyński TJ. Bernadzki KM, et al. Sci Rep. 2017 Aug 22;7(1):9116. doi: 10.1038/s41598-017-09590-7. Sci Rep. 2017. PMID: 28831123 Free PMC article. - To build a synapse: signaling pathways in neuromuscular junction assembly.
Wu H, Xiong WC, Mei L. Wu H, et al. Development. 2010 Apr;137(7):1017-33. doi: 10.1242/dev.038711. Development. 2010. PMID: 20215342 Free PMC article. Review.
References
- Apel E.D., Glass D.J., Moscoso L.M., Yancopoulos G.D., Sanes J.R. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 1997;4:623–635. - PubMed
- Apel E.D., Lewis R.M., Grady R.M., Sanes J.R. Syne-1a dystrophin- and klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J. Biol. Chem. 2000;275:31986–31995. - PubMed
- Brakeman P.R., Lanahan A.A., O'Brien R., Roche K., Barnes C.A., Huganir R.L., Worley P.F. Homera protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. - PubMed
- Burden S.J., DePalma R.L., Gottesman G.S. Crosslinking of proteins in acetylcholine receptor-rich membranesassociation between the beta-subunit and the 43 kd subsynaptic protein. Cell. 1983;35:687–692. - PubMed
- Cartaud A., Coutant S., Petrucci T.C., Cartaud J. Evidence for in situ and in vitro association between beta-dystroglycan and the subsynaptic 43K rapsyn protein. Consequence for acetylcholine receptor clustering at the synapse. J. Biol. Chem. 1998;273:11321–11326. - PubMed