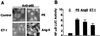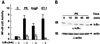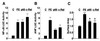Activation of NF-kappa B is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes - PubMed (original) (raw)
Activation of NF-kappa B is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes
N H Purcell et al. Proc Natl Acad Sci U S A. 2001.
Abstract
The transcription factor NF-kappaB regulates expression of genes that are involved in inflammation, immune response, viral infection, cell survival, and division. However, the role of NF-kappaB in hypertrophic growth of terminally differentiated cardiomyocytes is unknown. Here we report that NF-kappaB activation is required for hypertrophic growth of cardiomyocytes. In cultured rat primary neonatal ventricular cardiomyocytes, the nuclear translocation of NF-kappaB and its transcriptional activity were stimulated by several hypertrophic agonists, including phenylephrine, endothelin-1, and angiotensin II. The activation of NF-kappaB was inhibited by expression of a "supersuppressor" IkappaBalpha mutant that is resistant to stimulation-induced degradation and a dominant negative IkappaB kinase (IKKbeta) mutant that can no longer be activated by phosphorylation. Furthermore, treatment with phenylephrine induced IkappaBalpha degradation in an IKK-dependent manner, suggesting that NF-kappaB is a downstream target of the hypertrophic agonists. Importantly, expression of the supersuppressor IkappaBalpha mutant or the dominant negative IKKbeta mutant blocked the hypertrophic agonist-induced expression of the embryonic gene atrial natriuretic factor and enlargement of cardiomyocytes. Conversely, overexpression of NF-kappaB itself induced atrial natriuretic factor expression and cardiomyocyte enlargement. These findings suggest that NF-kappaB plays a critical role in the hypertrophic growth of cardiomyocytes and may serve as a potential target for the intervention of heart disease.
Figures
Figure 1
Stimulation of NF-κB activity by various hypertrophic agonists in primary cardiomyocytes. (A) Cardiomyocytes grown on coverslips were serum-starved for 24 h before treatment with PE (50 μM), ET-1 (50 nM), and AngII (50 μM) for 30 min or left untreated. Cells were fixed, blocked, and stained with anti-p65 mAb followed by donkey anti-mouse FITC (The Jackson Laboratory). The nuclear translocation of p65/RelA was visualized by using an Olympus fluorescence microscope. (B) Primary cardiomyocytes were transfected with the 2 × NF-κB reporter plasmid (0.25 μg/plate) and treated with PE (50 μM), AngII (50 μM), and ET-1 (50 nM) for 24 h or left untreated. Cells were harvested, and the Luc activity was determined and normalized to the protein content of each extract. Luc activity expressed by cells transfected with empty vector was given an arbitrary value of 1. The results are presented as the mean ± SE and represent four individual experiments.
Figure 2
NF-κB activation in cardiomyocytes involves IκBα degradation. (A) Primary cardiomyocytes were transfected with the 2 × NF-κB reporter plasmid (0.25 μg/plate), with or without the supersuppressor IκBα (32Ala/36Ala) mutant (50 ng/plate). Cells were serum-starved and then treated with PE (50 μM), AngII (50 μM), and ET-1 (50 nM) or left untreated. After 24 h, cells were harvested and the Luc activity was determined as described in Fig. 1. The results are presented as the mean ± SE and represent three individual experiments. (B) Primary cardiomyocytes were serum-starved and treated with PE for the indicated times or left untreated. Cells were harvested, and cell extracts (50 μg) were analyzed for degradation of IκBα proteins by immunoblotting with an anti-IκBα mAb (Santa Cruz Biotechnology). The protein level of endogenous actin also was analyzed to serve as a control.
Figure 3
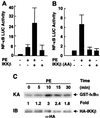
Activation of NF-κB by the hypertrophic agonist PE is mediated by the IKK complex. Primary cardiomyocytes were cotransfected with the 2 × NF-κB-Luc reporter plasmid (0.25 μg/plate) and expression vectors encoding HA-IKKβ (A) (100 ng/plate) or the dominant negative HA-IKKβ (177Ala/181Ala) (10 ng/plate), or empty vector, as indicated. Cells were treated with 10 μM PE or left untreated. After 24 h, cells were harvested, and the Luc activity was determined as described for Fig. 1. The results are presented as the mean ± SE and represent five individual experiments. (C) Primary cardiomyocytes were transfected with the expression vector encoding HA-IKKβ (3 μg/plate) or empty vector. After 40 h, cells were treated with PE (50 μM) for various times, or left untreated, as indicated. HA-IKKβ was immunoprecipitated, and its activity was determined by immunocomplex kinase assays with glutathione _S_-transferase-IκBα as a substrate, as described (31). Substrate phosphorylation was quantitated with a PhosphorImager. Fold stimulation is indicated. (Lower) An aliquot of each lysate was analyzed for its content of HA-IKKβ by immunoblotting, as described (31). ns, nonspecific.
Figure 4
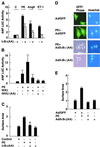
Inhibition of NF-κB activation blocks the hypertrophic effects of PE, ET-1, and AngII on cardiomyocytes. Primary cardiomyocytes were transfected with the ANF-Luc reporter plasmid (1 μg/plate), with or without (A) the supersuppressor HA-IκBα (32Ala/36Ala) mutant (50 ng/plate), (B) wild-type HA-IKKβ (10 ng/plate), or the dominant negative HA-IKKβ (177Ala/181Ala) mutant (10 ng/plate). Cells were treated with PE (50 μM), ET-1 (50 nM), and AngII (50 μM) or left untreated. After 24 h, cells were harvested and the Luc activity was determined as described in Fig. 1. The results are presented as the mean ± SE and represent four individual experiments. (C) Primary cardiomyocytes grown on coverslips were transfected with the expression vector encoding the dominant negative HA-IκBα (32Ala/36Ala) mutant and treated with PE (50 μM). After 4 days, transfected cells were identified by indirect immunofluorescence with anti-HA mAb (Covance, Richmond, CA). Cells were visualized by using an Olympus fluorescence microscope, and the surface area of cells were measured by using
scion imaging
software. *,P < 0.02. (D and E) Primary cardiomyocytes grown on coverslips were infected with Ads encoding the dominant negative IκBα (32Ala/36Ala) mutant or GFP at a multiplicity of infection of 100. After 16 h, cells were treated with or without PE (50 μM). Cell surface areas were measured 4 days later as described in C. The data were from three individual experiments with n = 60 cells, presented as mean ± SE. *, P < 0.02.
Figure 5
Overexpression of members of the NF-κB family, p65 and c-Rel, induces ANF expression and cell enlargement in cardiomyocytes. Primary cardiomyocytes were cotransfected with the 2 × NF-κB-Luc reporter plasmid (A) (0.25 μg/plate) or the ANF-Luc reporter plasmid (B) (1 μg/plate) with the expression vector encoding p65 or c-Rel (50 ng each), or empty vector, as indicated. After 24 h, cells were harvested and the Luc activity was determined as described for Fig. 1. The results are presented as the mean ± SE and represent five individual experiments. (C) Primary cardiomyocytes grown on coverslips were transfected with the expression vector encoding HA-p65 or c-Rel (2 μg each). After 4 days, transfected cells were identified by indirect immunofluorescence with anti-HA mAb (Covance) or anti-c-Rel mAb, respectively (Santa Cruz Biotechnology). Cells were visualized by using an Olympus fluorescence microscope, and the surface area of cells were measured by using
scion imaging
software. The results are presented as mean ± SE and represent three individual experiments with n = 30. *,P < 0.01.
Similar articles
- Involvement of nuclear factor-kappaB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy.
Hirotani S, Otsu K, Nishida K, Higuchi Y, Morita T, Nakayama H, Yamaguchi O, Mano T, Matsumura Y, Ueno H, Tada M, Hori M. Hirotani S, et al. Circulation. 2002 Jan 29;105(4):509-15. doi: 10.1161/hc0402.102863. Circulation. 2002. PMID: 11815436 - Activation of nuclear factor-kappaB is necessary for myotrophin-induced cardiac hypertrophy.
Gupta S, Purcell NH, Lin A, Sen S. Gupta S, et al. J Cell Biol. 2002 Dec 23;159(6):1019-28. doi: 10.1083/jcb.200207149. Epub 2002 Dec 16. J Cell Biol. 2002. PMID: 12486112 Free PMC article. - A20 is dynamically regulated in the heart and inhibits the hypertrophic response.
Cook SA, Novikov MS, Ahn Y, Matsui T, Rosenzweig A. Cook SA, et al. Circulation. 2003 Aug 12;108(6):664-7. doi: 10.1161/01.CIR.0000086978.95976.41. Epub 2003 Aug 4. Circulation. 2003. PMID: 12900338 - Regulation and function of IKK and IKK-related kinases.
Häcker H, Karin M. Häcker H, et al. Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review. - Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.
Karin M, Ben-Neriah Y. Karin M, et al. Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
- Thioredoxin and ventricular remodeling.
Ago T, Sadoshima J. Ago T, et al. J Mol Cell Cardiol. 2006 Nov;41(5):762-73. doi: 10.1016/j.yjmcc.2006.08.006. Epub 2006 Sep 26. J Mol Cell Cardiol. 2006. PMID: 17007870 Free PMC article. Review. - Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling.
Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Braz JC, et al. J Clin Invest. 2003 May;111(10):1475-86. doi: 10.1172/JCI17295. J Clin Invest. 2003. PMID: 12750397 Free PMC article. - Nuclear factor-kappaB: its role in health and disease.
Kumar A, Takada Y, Boriek AM, Aggarwal BB. Kumar A, et al. J Mol Med (Berl). 2004 Jul;82(7):434-48. doi: 10.1007/s00109-004-0555-y. Epub 2004 Jun 3. J Mol Med (Berl). 2004. PMID: 15175863 Review. - Nelumbo nucifera Receptaculum Extract Suppresses Angiotensin II-Induced Cardiomyocyte Hypertrophy.
Cho S, Cho HW, Woo KW, Jeong J, Lim J, Park S, Seo M, Lim S. Cho S, et al. Molecules. 2019 Apr 26;24(9):1647. doi: 10.3390/molecules24091647. Molecules. 2019. PMID: 31027372 Free PMC article. - Proteasome inhibition decreases cardiac remodeling after initiation of pressure overload.
Hedhli N, Lizano P, Hong C, Fritzky LF, Dhar SK, Liu H, Tian Y, Gao S, Madura K, Vatner SF, Depre C. Hedhli N, et al. Am J Physiol Heart Circ Physiol. 2008 Oct;295(4):H1385-93. doi: 10.1152/ajpheart.00532.2008. Epub 2008 Aug 1. Am J Physiol Heart Circ Physiol. 2008. PMID: 18676687 Free PMC article.
References
- Baldwin A S. Annu Rev Immunol. 1996;14:649–683. - PubMed
- Baeuerle P A, Baltimore D. Cell. 1996;87:13–20. - PubMed
- Barnes P J, Karin M. N Engl J Med. 1997;336:1066–1071. - PubMed
- Thanos D, Maniatis T. Cell. 1995;80:529–532. - PubMed
- Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Genes Dev. 1995;9:2723–2735. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources