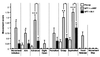Functional switch between motor tracts in the presence of the mAb IN-1 in the adult rat - PubMed (original) (raw)
Functional switch between motor tracts in the presence of the mAb IN-1 in the adult rat
O Raineteau et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Fine finger and hand movements in humans, monkeys, and rats are under the direct control of the corticospinal tract (CST). CST lesions lead to severe, long-term deficits of precision movements. We transected completely both CSTs in adult rats and treated the animals for 2 weeks with an antibody that neutralized the central nervous system neurite growth inhibitory protein Nogo-A (mAb IN-1). Anatomical studies of the rubrospinal tracts showed that the number of collaterals innervating the cervical spinal cord doubled in the mAb IN-1- but not in the control antibody-treated animals. Precision movements of the forelimb and fingers were severely impaired in the controls, but almost completely recovered in the mAb IN-1-treated rats. Low threshold microstimulation of the motor cortex induced a rapid forelimb electromyography response that was mediated by the red nucleus in the mAb IN-1 animals but not in the controls. These findings demonstrate an unexpectedly high capacity of the adult central nervous system motor system to sprout and reorganize in a targeted and functionally meaningful way.
Figures
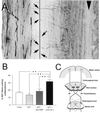
Figure 1
RST collateral formation after bPT. (A) Photomicrograph of a horizontal section of the rostral cervical spinal cord of a rat in which both CSTs have been transected and the RST traced. Descending RST fibers in the dorsolateral funiculus make collaterals (arrows) projecting to the gray matter where they arborize. Arrowhead indicates the midline. To measure the amount of collaterals emerging from the RST, all fibers crossing a line positioned 450 μm from the section border were counted and normalized as described in Materials and Methods. (B) Quantitative analysis showing the percentage of RST fibers making a collateral on a length of spinal cord of 1 mm. Unles, unlesioned rats, n = 6; bPT, lesioned rats, n = 7; bPT + anti-HRP, lesioned rats treated with the control antibody anti-HRP, n = 9; bPT + mAb IN-1, lesioned rats treated with the mAb IN-1,n = 12. **, P < 0.01. (Bars = +SEM.) (C) Scheme of the cortico- and rubrospinal projections and the lesion sites.
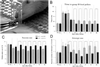
Figure 2
Food pellet-reaching task. (A) Rat grasping small food pellets stabilized with a forceps through a central opening in the wall of a transparent Plexiglas box. (B_–_D) Time course of recovery for the time (in sec) needed to grasp 10 pellets (B), the success rate in grasping 10 pellets (n = number of pellets grasped and eaten) (C), and the maximum number of attempts (n) to grasp a pellet in 10 trials (D). *, P < 0.05; **,P < 0.01; ***,P < 0.001. (Bars = +SEM.)
Figure 3
Video analysis of movement components. Individual movement components on day 14 after operation. A score of 0 indicates normal forelimb performance; 3 indicates an absence of movements. Asterisks on top of error bars indicate significance compared with preoperative values; asterisks on the very top indicate a comparison between the two experimental groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (Bars = +SEM.)
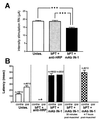
Figure 4
Electrophysiological assessment of the cortico-rubro-spinal pathway reorganization. (A) Red nucleus thresholds of stimulation to obtain a peripheral EMG activity in the forearm muscles (extensor carpi radialis and extensor digitorum communis). Unles., unlesioned rats,n = 5; bPT + anti-HRP, lesioned rats treated with the control antibody against HRP, n = 9; bPT + IN-1: lesioned rats treated with the mAb IN-1, n =11. ***, p < 0.001. (Bars, +SEM.) (B) EMG latencies for forearm muscles elicited by ICMS of the forelimb area in unlesioned, lesioned control-antibody (anti-HRP Ab), and lesioned, mAb IN-1-treated rats. ICMS in normal animals (5 rats, 2 hemicortex stimulations per rat) resulted in fast contralateral responses. An ipsilateral EMG response with a prolonged delay could also be found, but only at higher stimulation intensity. ICMS of the lesioned animals treated with the control antibody never resulted in movements (n = 9). In rats lesioned and treated with the mAb IN-1 (n = 11), ICMS led to contralateral EMG activity of increased latency. In two cases, an ipsilateral EMG response of comparable latency occurred. Muscimol injections into both red nuclei led to a loss of EMG activity. At 4–7 h after muscimol injection, ICMS resulted again in peripheral EMG activity. Unles., unlesioned rats; bPT + anti-HRP, lesioned rats treated with the control antibody against HRP; bPT + IN-1: lesioned rats treated with the mAb IN-1. (Bars, +SEM.)
Similar articles
- Rewiring of the corticospinal tract in the adult rat after unilateral stroke and anti-Nogo-A therapy.
Lindau NT, Bänninger BJ, Gullo M, Good NA, Bachmann LC, Starkey ML, Schwab ME. Lindau NT, et al. Brain. 2014 Mar;137(Pt 3):739-56. doi: 10.1093/brain/awt336. Epub 2013 Dec 18. Brain. 2014. PMID: 24355710 Clinical Trial. - Reorganization of descending motor tracts in the rat spinal cord.
Raineteau O, Fouad K, Bareyre FM, Schwab ME. Raineteau O, et al. Eur J Neurosci. 2002 Nov;16(9):1761-71. doi: 10.1046/j.1460-9568.2002.02243.x. Eur J Neurosci. 2002. PMID: 12431229 - Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats.
Seymour AB, Andrews EM, Tsai SY, Markus TM, Bollnow MR, Brenneman MM, O'Brien TE, Castro AJ, Schwab ME, Kartje GL. Seymour AB, et al. J Cereb Blood Flow Metab. 2005 Oct;25(10):1366-75. doi: 10.1038/sj.jcbfm.9600134. J Cereb Blood Flow Metab. 2005. PMID: 15889044 - [Functional role of the red nucleus in the cerebral cortex-cerebellum-spinal cord communication system].
Fanardzhian VV. Fanardzhian VV. Usp Fiziol Nauk. 2001 Apr-Jun;32(2):3-15. Usp Fiziol Nauk. 2001. PMID: 11548591 Review. Russian. - Nogo-A, a potent inhibitor of neurite outgrowth and regeneration.
Huber AB, Schwab ME. Huber AB, et al. Biol Chem. 2000 May-Jun;381(5-6):407-19. doi: 10.1515/BC.2000.053. Biol Chem. 2000. PMID: 10937871 Review.
Cited by
- Plasticity of intact rubral projections mediates spontaneous recovery of function after corticospinal tract injury.
Siegel CS, Fink KL, Strittmatter SM, Cafferty WB. Siegel CS, et al. J Neurosci. 2015 Jan 28;35(4):1443-57. doi: 10.1523/JNEUROSCI.3713-14.2015. J Neurosci. 2015. PMID: 25632122 Free PMC article. - Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury.
Li S, Strittmatter SM. Li S, et al. J Neurosci. 2003 May 15;23(10):4219-27. doi: 10.1523/JNEUROSCI.23-10-04219.2003. J Neurosci. 2003. PMID: 12764110 Free PMC article. - Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord.
Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Fouad K, et al. J Neurosci. 2005 Feb 2;25(5):1169-78. doi: 10.1523/JNEUROSCI.3562-04.2005. J Neurosci. 2005. PMID: 15689553 Free PMC article. - Who is who after spinal cord injury and repair? Can the brain stem descending motor pathways take control of skilled hand motor function?
García-Alías G, Edgerton VR. García-Alías G, et al. Neural Regen Res. 2015 Nov;10(11):1735-6. doi: 10.4103/1673-5374.165318. Neural Regen Res. 2015. PMID: 26807096 Free PMC article. No abstract available. - Rho signaling pathway targeted to promote spinal cord repair.
Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Dergham P, et al. J Neurosci. 2002 Aug 1;22(15):6570-7. doi: 10.1523/JNEUROSCI.22-15-06570.2002. J Neurosci. 2002. PMID: 12151536 Free PMC article.
References
- Little J W, Ditunno J F, Jr, Stiens S A, Harris R M. Arch Phys Med Rehab. 1999;80:587–599. - PubMed
- Bishop B. Phys Ther. 1982;62:1442–1451. - PubMed
- Basso D M, Beattie M S, Bresnahan J C. Exp Neurol. 1996;139:244–256. - PubMed
- Noble L J, Wrathall J R. Exp Neurol. 1989;103:34–40. - PubMed
- Kapfhammer J P, Schwab M E. J Comp Neurol. 1994;340:194–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources