Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes - PubMed (original) (raw)
Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes
E González-Suárez et al. EMBO J. 2001.
Abstract
Telomerase transgenics are an important tool to assess the role of telomerase in cancer, as well as to evaluate the potential use of telomerase for gene therapy of age-associated diseases. Here, we have targeted the expression of the catalytic component of mouse telomerase, mTERT, to basal keratinocytes using the bovine keratin 5 promoter. These telomerase-transgenic mice are viable and show histologically normal stratified epithelia with high levels of telomerase activity and normal telomere length. Interestingly, the epidermis of these mice is highly responsive to the mitogenic effects of phorbol esters, and it is more susceptible than that of wild-type littermates to the development skin tumors upon chemical carcinogenesis. The epidermis of telomerase-transgenic mice also shows an increased wound-healing rate compared with wild-type littermates. These results suggest that, contrary to the general assumption, telomerase actively promotes proliferation in cells that have sufficiently long telomeres and unravel potential risks of gene therapy for age-associated diseases based on telomerase upregulation.
Figures
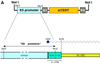
Fig. 1. Generation of K5-mTERT mice. (A) A scheme of the K5-mTERT transgene construct. The functional elements include a _Sal_I–_Nru_I fragment with the bovine K5 regulatory sequences (K5 promoter box), the rabbit β-globin intron 2 (G1 box), the coding sequence of the mTERT gene (mTERT box) and the SV40 early gene poly(A) addition signal (PA box). (B) Telomerase TRAP activity in the tail of wild-type and K5-mTERT founders (T1–T8). The two founders chosen for the study (T1 and T8) are highlighted in red. The asterisk highlights founder mouse T1, which was used for most of the experiments described here. (C) Telomerase TRAP activity in wild-type and K5-mTERT (T1) tissues. The indicated protein concentrations of the S-100 extract were used. Extracts were pre-treated (+) or not (–) with RNase A.
Fig. 1. Generation of K5-mTERT mice. (A) A scheme of the K5-mTERT transgene construct. The functional elements include a _Sal_I–_Nru_I fragment with the bovine K5 regulatory sequences (K5 promoter box), the rabbit β-globin intron 2 (G1 box), the coding sequence of the mTERT gene (mTERT box) and the SV40 early gene poly(A) addition signal (PA box). (B) Telomerase TRAP activity in the tail of wild-type and K5-mTERT founders (T1–T8). The two founders chosen for the study (T1 and T8) are highlighted in red. The asterisk highlights founder mouse T1, which was used for most of the experiments described here. (C) Telomerase TRAP activity in wild-type and K5-mTERT (T1) tissues. The indicated protein concentrations of the S-100 extract were used. Extracts were pre-treated (+) or not (–) with RNase A.
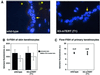
Fig. 2. Telomere fluorescence in skin sections. (A) Illustrative images showing telomere fluorescence in wild-type and K5-mTERT (T1) skin sections. The basal layer of skin keratinocytes is indicated (yellow arrow). (B) Quantification of telomere fluorescence on untreated skin sections or on skin sections treated with DMBA + TPA, as indicated. More than 50 keratinocyte nuclei of each genotype were analyzed by Q-FISH. (C) Average telomere fluorescence as determined by Flow-FISH of three wild-type and six K5-mTERT primary keratinocyte cultures. Between 2000 and 3000 nuclei were analyzed by flow cytometry for each culture.
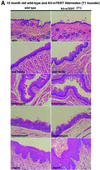
Fig. 3. (A and B) Histology of stratified epithelia in littermate 15- to 19-month-old wild-type and K5-mTERT (T1 or T8, as indicated) transgenics. The epithelia studied were skin, oral cavity, esophagus, forestomach and vagina. No significant histological differences between genotypes were observed. Magnification ×20.
Fig. 3. (A and B) Histology of stratified epithelia in littermate 15- to 19-month-old wild-type and K5-mTERT (T1 or T8, as indicated) transgenics. The epithelia studied were skin, oral cavity, esophagus, forestomach and vagina. No significant histological differences between genotypes were observed. Magnification ×20.
Fig. 4. (A) The total numbers of papillomas are plotted versus the number of weeks after the start of carcinogen treatment. Termination of TPA treatment (week 15) is indicated by an asterisk. (B) Average number of papillomas per mouse at week 15 after the start of carcinogen treatment. Wild-type and K5-mTERT (T1) transgenics showed an average of 9.9 ± 6.3 and 20.0 ± 9.8 papillomas per mouse, respectively. (C) Survival of DMBA + TPA-treated wild-type and K5-mTERT (T1) mice during the multistage chemical carcinogenesis experiment.
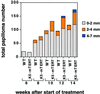
Fig. 5. The total numbers of papillomas of different sizes are plotted versus the number of weeks after the start of DMBA + TPA treatment in wild-type and K5-mTERT (T8) transgenics. The total number of mice is nine each for wild-type and K5-mTERT genotypes (T8).
Fig. 6. Histopathology of skin lesions. (A) Both DMBA + TPA-treated wild-type and K5-mTERT (T1) mice show typical papilloma lesions in the skin (see ‘papilloma’). Seven weeks after termination of DMBA + TPA treatment, the DMBA + TPA-treated K5-mTERT (T1) mice show skin with severe hyperplasia (see ‘hyperplastic skin’); this is not observed in the skin of similarly treated wild-type mice (see ‘normal skin’). A forestomach lesion showing marked hyperplasia and hyperkeratosis corresponding to a DMBA + TPA-treated K5-mTERT (T1) mouse is shown (see ‘abnormal forestomach’); these lesions were never detected in similary treated wild-type mice (see ‘normal forestomach’). Magnification ×20. (B) Skin lesions in wild-type and K5-mTERT (T1) trangenics after repeated weekly exposure to TPA (no DMBA). Incipient papilloma lesions are shown for TPA-treated K5-mTERT (T1) mice (see ‘incipient papilloma’); these lesions were never detected in similarly treated wild-type mice. A hyperplastic esophagus lesion is shown for TPA-treated K5-mTERT (T1) mice (see ‘hyperplastic esophagus’); these lesions were never found in similarly treated wild-type mice. Magnification ×20.
Fig. 6. Histopathology of skin lesions. (A) Both DMBA + TPA-treated wild-type and K5-mTERT (T1) mice show typical papilloma lesions in the skin (see ‘papilloma’). Seven weeks after termination of DMBA + TPA treatment, the DMBA + TPA-treated K5-mTERT (T1) mice show skin with severe hyperplasia (see ‘hyperplastic skin’); this is not observed in the skin of similarly treated wild-type mice (see ‘normal skin’). A forestomach lesion showing marked hyperplasia and hyperkeratosis corresponding to a DMBA + TPA-treated K5-mTERT (T1) mouse is shown (see ‘abnormal forestomach’); these lesions were never detected in similary treated wild-type mice (see ‘normal forestomach’). Magnification ×20. (B) Skin lesions in wild-type and K5-mTERT (T1) trangenics after repeated weekly exposure to TPA (no DMBA). Incipient papilloma lesions are shown for TPA-treated K5-mTERT (T1) mice (see ‘incipient papilloma’); these lesions were never detected in similarly treated wild-type mice. A hyperplastic esophagus lesion is shown for TPA-treated K5-mTERT (T1) mice (see ‘hyperplastic esophagus’); these lesions were never found in similarly treated wild-type mice. Magnification ×20.
Fig. 7. Wound healing of wild-type and K5-mTERT (T1) skin. (A) Percentage of initial wound area left 3 and 4 days after the punch was created in a group of six wild-type and six K5-mTERT (T1) mice. Statistical analysis showing that the healing rate is significantly different between genotypes is also shown (_P_-values). (B and C) Rate of wound healing in two wild-type and K5-TERT (T1) littermate pairs; the results are presented as the percentage of open wound area versus time after wound creation. Closed circles, wild-type mice; open circles, K5-mTERT (T1) transgenics. (D) Gross appearance of healing wounds in a wild-type and a K5-mTERT (T1) mouse 5 and 2 days, for wounds 1 and 2, respectively, after wound creation. Just prior to sacrifice of the mice, a third wound (wound 3) was created as an indicator of initial wound areas.
Fig. 7. Wound healing of wild-type and K5-mTERT (T1) skin. (A) Percentage of initial wound area left 3 and 4 days after the punch was created in a group of six wild-type and six K5-mTERT (T1) mice. Statistical analysis showing that the healing rate is significantly different between genotypes is also shown (_P_-values). (B and C) Rate of wound healing in two wild-type and K5-TERT (T1) littermate pairs; the results are presented as the percentage of open wound area versus time after wound creation. Closed circles, wild-type mice; open circles, K5-mTERT (T1) transgenics. (D) Gross appearance of healing wounds in a wild-type and a K5-mTERT (T1) mouse 5 and 2 days, for wounds 1 and 2, respectively, after wound creation. Just prior to sacrifice of the mice, a third wound (wound 3) was created as an indicator of initial wound areas.
Fig. 8. (A) p53 levels in wild-type and K5-mTERT (T1) skin keratinocytes. Immunohistochemistry with p53 antibody of untreated and DMBA + TPA-treated wild-type and K5-mTERT (T1) skin. The control untreated wild-type and K5-mTERT (T1) skin show similar p53 positivity in basal keratinocyte nuclei; p53–/– mouse skin is shown as a negative control. Wild-type and K5-mTERT (T1) papillomas show decreased p53 staining of basal keratinocytes compared with wild-type skin. Magnification ×20. (B) Ras protein levels detected by western blot in wild-type and K5-mTERT (T1) primary keratinocytes. No significant differences in Ras protein levels were detected between genotypes. As a control for loading, nuclear protein Ku70 was also detected. Different numbers indicate different primary keratinocyte cultures. The arrows indicate the positions of theRas- and Ku86-specific bands. (C) c-myc mRNA levels detected by northern blot in the skin of four K5-mTERT (T1 or T8, as indicated) and the corresponding wild-type littermate skins. Mice 30, 32, 34 and 35 are littermates derived from founder T8. Mice 130, 131, 132 and 134 are littermates derived from founder T1. As a positive control for c-myc detection, total RNA from interleukin-2-stimulated Ba/FO3 cells was used (Hatakeyama et al., 1989). As control for loading, actin was also detected in the blots. Table II shows quantification of c-myc levels.
Fig. 8. (A) p53 levels in wild-type and K5-mTERT (T1) skin keratinocytes. Immunohistochemistry with p53 antibody of untreated and DMBA + TPA-treated wild-type and K5-mTERT (T1) skin. The control untreated wild-type and K5-mTERT (T1) skin show similar p53 positivity in basal keratinocyte nuclei; p53–/– mouse skin is shown as a negative control. Wild-type and K5-mTERT (T1) papillomas show decreased p53 staining of basal keratinocytes compared with wild-type skin. Magnification ×20. (B) Ras protein levels detected by western blot in wild-type and K5-mTERT (T1) primary keratinocytes. No significant differences in Ras protein levels were detected between genotypes. As a control for loading, nuclear protein Ku70 was also detected. Different numbers indicate different primary keratinocyte cultures. The arrows indicate the positions of theRas- and Ku86-specific bands. (C) c-myc mRNA levels detected by northern blot in the skin of four K5-mTERT (T1 or T8, as indicated) and the corresponding wild-type littermate skins. Mice 30, 32, 34 and 35 are littermates derived from founder T8. Mice 130, 131, 132 and 134 are littermates derived from founder T1. As a positive control for c-myc detection, total RNA from interleukin-2-stimulated Ba/FO3 cells was used (Hatakeyama et al., 1989). As control for loading, actin was also detected in the blots. Table II shows quantification of c-myc levels.
Similar articles
- Constitutive expression of erbB2 in epidermis of transgenic mice results in epidermal hyperproliferation and spontaneous skin tumor development.
Kiguchi K, Bol D, Carbajal S, Beltrán L, Moats S, Chan K, Jorcano J, DiGiovanni J. Kiguchi K, et al. Oncogene. 2000 Aug 31;19(37):4243-54. doi: 10.1038/sj.onc.1203778. Oncogene. 2000. PMID: 10980598 - Increased skin carcinogenesis in a keratinocyte directed thioredoxin-1 transgenic mouse.
Mustacich D, Wagner A, Williams R, Bair W, Barbercheck L, Stratton SP, Bhattacharyya AK, Powis G. Mustacich D, et al. Carcinogenesis. 2004 Oct;25(10):1983-9. doi: 10.1093/carcin/bgh195. Epub 2004 May 27. Carcinogenesis. 2004. PMID: 15166090 - Overexpression of plasminogen activator inhibitor type 2 in basal keratinocytes enhances papilloma formation in transgenic mice.
Zhou HM, Bolon I, Nichols A, Wohlwend A, Vassalli JD. Zhou HM, et al. Cancer Res. 2001 Feb 1;61(3):970-6. Cancer Res. 2001. PMID: 11221892 - Skin aging: a role for telomerase and telomere dynamics?
Boukamp P. Boukamp P. Curr Mol Med. 2005 Mar;5(2):171-7. doi: 10.2174/1566524053586644. Curr Mol Med. 2005. PMID: 15974870 Review. - Mammalian telomerase: catalytic subunit and knockout mice.
Kipling D. Kipling D. Hum Mol Genet. 1997 Nov;6(12):1999-2004. doi: 10.1093/hmg/6.12.1999. Hum Mol Genet. 1997. PMID: 9328462 Review.
Cited by
- Telomerase extracurricular activities.
Chang S, DePinho RA. Chang S, et al. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):12520-2. doi: 10.1073/pnas.212514699. Epub 2002 Sep 23. Proc Natl Acad Sci U S A. 2002. PMID: 12271146 Free PMC article. No abstract available. - The role of telomeres and telomerase in cancer research.
Stewart SA, Bertuch AA. Stewart SA, et al. Cancer Res. 2010 Oct 1;70(19):7365-71. doi: 10.1158/0008-5472.CAN-10-1373. Epub 2010 Sep 14. Cancer Res. 2010. PMID: 20841475 Free PMC article. - WRAP53 promotes cancer cell survival and is a potential target for cancer therapy.
Mahmoudi S, Henriksson S, Farnebo L, Roberg K, Farnebo M. Mahmoudi S, et al. Cell Death Dis. 2011 Jan 13;2(1):e114. doi: 10.1038/cddis.2010.90. Cell Death Dis. 2011. PMID: 21368886 Free PMC article. - Supraphysiological protection from replication stress does not extend mammalian lifespan.
Albers E, Avram A, Sbroggio M, Fernandez-Capetillo O, Lopez-Contreras AJ. Albers E, et al. Aging (Albany NY). 2020 Apr 6;12(7):5612-5624. doi: 10.18632/aging.103039. Epub 2020 Apr 6. Aging (Albany NY). 2020. PMID: 32253367 Free PMC article. - Identification of PITX1 as a TERT suppressor gene located on human chromosome 5.
Qi DL, Ohhira T, Fujisaki C, Inoue T, Ohta T, Osaki M, Ohshiro E, Seko T, Aoki S, Oshimura M, Kugoh H. Qi DL, et al. Mol Cell Biol. 2011 Apr;31(8):1624-36. doi: 10.1128/MCB.00470-10. Epub 2011 Feb 7. Mol Cell Biol. 2011. PMID: 21300782 Free PMC article.
References
- Balmain A., Ramsden,M., Bowden,G.T. and Smith,J. (1984) Activation of the mouse Harvey-ras gene in chemically induced benign skin papillomas. Nature, 307, 658–660. - PubMed
- Balmain A. et al. (1992) Functional loss of tumor suppressor genes in multistage chemical carcinogenesis. In Harris,C.C. et al. (eds), Multistage Carcinogenesis. Japan Scientific Society Press/CRC Press, Boca Raton, FL, pp. 97–108.
- Bednarek A.K., Chu,Y., Slaga,T.J. and Aldaz,C.M. (1997). Telomerase and cell proliferation in mouse skin papillomas. Mol. Carcinog., 20, 329–310. - PubMed
- Blackburn E.H. (1991) Structure and function of telomeres. Nature, 350, 569–573. - PubMed
- Blasco M.A., Funk,W.D., Villeponteau,B. and Greider,C.W. (1995) Functional characterization and developmental regulation of mouse telomerase RNA. Science, 269, 1267–1270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials