Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses - PubMed (original) (raw)
Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses
J M McNally et al. J Virol. 2001 Jul.
Abstract
Experiments designed to distinguish virus-specific from non-virus-specific T cells showed that bystander T cells underwent apoptosis and substantial attrition in the wake of a strong T-cell response. Memory CD8 T cells (CD8(+) CD44(hi)) were most affected. During acute viral infection, transgenic T cells that were clearly defined as non-virus specific decreased in number and showed an increase in apoptosis. Also, use of lymphocytic choriomeningitis virus (LCMV) carrier mice, which lack LCMV-specific T cells, showed a significant decline in non-virus-specific memory CD8 T cells that correlated to an increase in apoptosis in response to the proliferation of adoptively transferred virus-specific T cells. Attrition of T cells early during infection correlated with the alpha/beta interferon (IFN-alpha/beta) peak, and the IFN inducer poly(I:C) caused apoptosis and attrition of CD8(+) CD44(hi) T cells in normal mice but not in IFN-alpha/beta receptor-deficient mice. Apoptotic attrition of bystander T cells may make room for the antigen-specific expansion of T cells during infection and may, in part, account for the loss of T-cell memory that occurs when the host undergoes subsequent infections.
Figures
FIG. 1
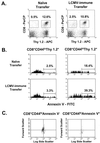
Adoptive transfer of LCMV-immune splenocytes into LCMV carrier mice leads to a reduction in the number of host CD8 T cells and an increase in host T-cell apoptosis. (A) Spleens from LCMV carrier mice (Thy 1.2+), which received an adoptive transfer of 3 × 107 naive or LCMV-immune Thy 1.1+ splenocytes (Thy 1.2−), were stained to determine the proportions of donor and host-derived CD8 T cells 6 days after reconstitution. APC, antigen-presenting cells. (B) Host-derived cells were gated on memory (CD44hi) and naive (CD44lo) phenotypes, and apoptosis of these populations was assessed using annexin V. (C) Light scatter properties of annexin V+ or annexin V− host-derived memory T cells is shown, demonstrating that the annexin V+ population is smaller and more granular than the annexin V− T cells. In all cases, data shown are from an individual mouse in a representative experiment (n = 3).
FIG. 2
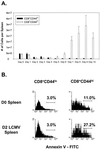
Kinetic analysis of CD8+ T cells found in the spleens of mice undergoing an acute LCMV infection. (A) Spleens from LCMV-infected B6 mice were analyzed using flow cytometry to determine the number of memory (CD44hi) or naive (CD44lo) CD8 T cells during the course of the viral infection. Results are expressed as the mean number of cells per spleen ± standard deviation (n = 3 per time point). (B) Annexin staining of memory and naive CD8 T cells from a representative mouse 2 days following LCMV infection. In both panels, gating on CD44hi versus CD44lo cells was determined using the day 9 postinfection time point to include the majority of activated cells. This resulted in a broader definition of CD44hi cells than would occur if day 0 control mice were used for determining the gates. D0, day 0; D2, day 2.
FIG. 3
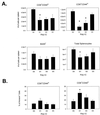
Poly(I:C) injection leads to a significant decrease in the number of CD8+ CD44hi T cells in the spleen and an increase in their apoptosis. (A) Splenocytes from B6 mice injected with poly(I:C) were stained for CD8, CD44, and B220 expression to determine the number of CD8 T cells (memory [CD44hi] or naive [CD44lo]) and B cells present on days 1 to 3 postinjection. The proportion of annexin V+ cells for these mice is shown in panel B. Data shown are from a representative experiment (n = 5). D0, day 0; D1, day 1; etc. *, P < 0.05, compared to day 0 control.
FIG. 4
Poly(I:C) induces attrition of LCMV-specific memory CD8 T cells. Splenocytes from LCMV-immune mice (see Materials and Methods) were analyzed for expression of CD8 and an MHC tetramer (H-2Db) specific for the LCMV peptide NP396–404. LCMV-immune mice were injected with poly(I:C) and analyzed, as previously described. Representative histograms from an experiment performed twice are shown. D0, day 0; D1, day 1.
FIG. 5
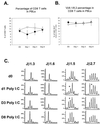
The T-cell repertoire is not permanently altered by poly(I:C) treatment, as determined by FACS analysis and CDR3 length spectratyping. (A and B) Sequentially bled LCMV-immune mice were analyzed following poly(I:C) treatment to determine if any significant changes in the T-cell repertoire took place as a result of the attrition of memory T cells. Although the percentage of CD8 T cells is reduced on day 1 following poly(I:C) injection (A), the proportion of those cells that are Vβ 8.1/8.2+ was not altered (B). ▿, control mice; ●, poly(I:C)-treated mice. (C) The Vβ8.1 T cells were further analyzed for changes in their repertoire using CDR3 length spectratyping. Mice exhibiting the most significant changes in T-cell repertoire for Jβ1.3, Jβ1.6, Jβ1.5, and Jβ2.7 are shown. Even though these mice exhibited the most significant changes of all mice and Jβ's tested, the changes were transient and the mice returned to their original distribution by 8 days after the poly(I:C) treatment. *, change in spectratype. PBLs, peripheral blood lymphocytes; d0 and D0, day 0; d1, day 1; D3, day 3; D8, day 8.
Similar articles
- Bystander sensitization to activation-induced cell death as a mechanism of virus-induced immune suppression.
Zarozinski CC, McNally JM, Lohman BL, Daniels KA, Welsh RM. Zarozinski CC, et al. J Virol. 2000 Apr;74(8):3650-8. doi: 10.1128/jvi.74.8.3650-3658.2000. J Virol. 2000. PMID: 10729141 Free PMC article. - Aplastic anemia rescued by exhaustion of cytokine-secreting CD8+ T cells in persistent infection with lymphocytic choriomeningitis virus.
Binder D, van den Broek MF, Kägi D, Bluethmann H, Fehr J, Hengartner H, Zinkernagel RM. Binder D, et al. J Exp Med. 1998 Jun 1;187(11):1903-20. doi: 10.1084/jem.187.11.1903. J Exp Med. 1998. PMID: 9607930 Free PMC article. - IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections.
Bahl K, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Selin LK, Welsh RM. Bahl K, et al. J Immunol. 2006 Apr 1;176(7):4284-95. doi: 10.4049/jimmunol.176.7.4284. J Immunol. 2006. PMID: 16547266 - Viruses and T cell turnover: evidence for bystander proliferation.
Tough DF, Sprent J. Tough DF, et al. Immunol Rev. 1996 Apr;150:129-42. doi: 10.1111/j.1600-065x.1996.tb00699.x. Immunol Rev. 1996. PMID: 8782705 Review. No abstract available. - Bystander stimulation of T cells in vivo by cytokines.
Tough DF, Sprent J. Tough DF, et al. Vet Immunol Immunopathol. 1998 May 15;63(1-2):123-9. doi: 10.1016/s0165-2427(98)00088-9. Vet Immunol Immunopathol. 1998. PMID: 9656447 Review.
Cited by
- Type I interferons in infectious disease.
McNab F, Mayer-Barber K, Sher A, Wack A, O'Garra A. McNab F, et al. Nat Rev Immunol. 2015 Feb;15(2):87-103. doi: 10.1038/nri3787. Nat Rev Immunol. 2015. PMID: 25614319 Free PMC article. Review. - CD122+CD8+ Treg suppress vaccine-induced antitumor immune responses in lymphodepleted mice.
Wang LX, Li Y, Yang G, Pang PY, Haley D, Walker EB, Urba WJ, Hu HM. Wang LX, et al. Eur J Immunol. 2010 May;40(5):1375-85. doi: 10.1002/eji.200839210. Eur J Immunol. 2010. PMID: 20186876 Free PMC article. - Analysis of apoptosis of memory T cells and dendritic cells during the early stages of viral infection or exposure to toll-like receptor agonists.
Bahl K, Hüebner A, Davis RJ, Welsh RM. Bahl K, et al. J Virol. 2010 May;84(10):4866-77. doi: 10.1128/JVI.02571-09. Epub 2010 Mar 3. J Virol. 2010. PMID: 20200235 Free PMC article. - Acute induction of cell death-related IFN stimulated genes (ISG) differentiates highly from moderately virulent CSFV strains.
Renson P, Blanchard Y, Le Dimna M, Felix H, Cariolet R, Jestin A, Le Potier MF. Renson P, et al. Vet Res. 2010 Jan-Feb;41(1):7. doi: 10.1051/vetres/2009055. Epub 2009 Oct 1. Vet Res. 2010. PMID: 19793538 Free PMC article. - IL-15-Independent Maintenance of Tissue-Resident and Boosted Effector Memory CD8 T Cells.
Schenkel JM, Fraser KA, Casey KA, Beura LK, Pauken KE, Vezys V, Masopust D. Schenkel JM, et al. J Immunol. 2016 May 1;196(9):3920-6. doi: 10.4049/jimmunol.1502337. Epub 2016 Mar 21. J Immunol. 2016. PMID: 27001957 Free PMC article.
References
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relationship. Science. 1996;272:54–60. - PubMed
- Biron C A, Turgiss L R, Welsh R M. Increase in NK cell number and turnover rate during acute viral infection. J Immunol. 1983;131:1539–1545. - PubMed
- Biron C A, Welsh R M. Blastogenesis of natural killer cells during viral infection in vivo. J Immunol. 1982;129:2788–2795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR035506/AR/NIAMS NIH HHS/United States
- AI07272/AI/NIAID NIH HHS/United States
- P30 DK032520/DK/NIDDK NIH HHS/United States
- R37 AI017672/AI/NIAID NIH HHS/United States
- T32 AI007272/AI/NIAID NIH HHS/United States
- T32 AI007349/AI/NIAID NIH HHS/United States
- AR35506/AR/NIAMS NIH HHS/United States
- P30 DK 32520/DK/NIDDK NIH HHS/United States
- AI17672/AI/NIAID NIH HHS/United States
- R01 AI017672/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous