CD44 is a major E-selectin ligand on human hematopoietic progenitor cells - PubMed (original) (raw)
CD44 is a major E-selectin ligand on human hematopoietic progenitor cells
C J Dimitroff et al. J Cell Biol. 2001.
Abstract
E-selectin plays a critical role in mediating tissue-specific homing of T cells into skin, and of primitive hematopoietic progenitor cells (HPCs) into bone marrow (BM). Though it is known that a glycoform of PSGL-1 (CLA) functions as the principal E-selectin ligand on human T lymphocytes, the E-selectin ligand(s) of human HPCs has not been identified. We used a shear-based adherence assay to analyze and define the E-selectin ligand activity of membrane proteins from human HPCs. Our data show that PSGL-1 expressed on human HPCs is an E-selectin ligand, and that HPCs also express a previously unrecognized E-selectin ligand, CD44. The E-selectin ligand activity of CD44 is conferred by the elaboration of sialylated, fucosylated binding determinants on N-glycans. This glycoform of CD44 is expressed on primitive CD34+ human HPCs, but not on more mature hematopoietic cells. Under physiologic flow conditions, this molecule mediates E-selectin-dependent rolling interactions over a wider shear range than that of PSGL-1, and promotes human HPC rolling interactions on E-selectin expressed on human BM endothelial cells. These findings offer new insights into the structural biology and physiology of CD44, and into the molecular basis of E-selectin-dependent adhesive interactions that direct homing of human HPC to BM.
Figures
Figure 3
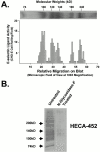
HECA-452–reactive CD44 functions as an E-selectin ligand. (A) KG1a membrane protein (10 μg) was resolved on a reducing 6% SDS-PAGE gel, blotted onto PVDF membrane, stained with HECA-452, and HECA-452 immunoblots blots were rendered transparent with 10% glycerol. CHO-E cells (2 × 106/ml) were then perfused over these blots at a defined shear stress of 3.8 dynes/cm2. Several HECA-452–stained bands from KG1a membrane protein–supported, E-selectin–dependent CHO-E cell rolling. (B) KG1a membrane proteins (10 μg) were treated with _N_-glycosidase F, separated on a reducing 6% SDS-PAGE gel, and immunostained with HECA-452. (C) Immunoprecipitated PSGL-1 was resolved on a reducing 6% SDS-PAGE gel and Western blotted with either HECA-452 (left) or anti–PSGL-1 antibody 4H10 (right). (lane 1) 10 μg of total KG1a membrane protein; (lane 2) immunoprecipitated PSGL-1 from 100 μg of KG1a membrane protein; (lane 3) 100 μg of total KG1a membrane protein, and (lane 4) immunoprecipitated PSGL-1, from 100 μg of KG1a membrane protein. Note that HECA-452–stained bands at 140 and 220 kD correspond to PSGL-1. (D) Isotype control or Hermes-1 immunoprecipitated CD44 from KG1a membrane protein (50 μg) was resolved on a reducing 9% SDS-PAGE gel and immunoblotted with HECA-452. Immunoprecipitated CD44 from KG1a membrane proteins (50 μg) treated with _N_-glycosidase F was also immunoblotted with HECA-452. Though CHO-E cell rolling frequencies are presented as the mean ± SD of E-selectin–mediated cell rolling at 3.8 dynes/cm2 measured on the 100-kD isoform of CD44, no CHO-E rolling was observed along the entire length of the _N_-glycosidase F–treated lane.
Figure 3
HECA-452–reactive CD44 functions as an E-selectin ligand. (A) KG1a membrane protein (10 μg) was resolved on a reducing 6% SDS-PAGE gel, blotted onto PVDF membrane, stained with HECA-452, and HECA-452 immunoblots blots were rendered transparent with 10% glycerol. CHO-E cells (2 × 106/ml) were then perfused over these blots at a defined shear stress of 3.8 dynes/cm2. Several HECA-452–stained bands from KG1a membrane protein–supported, E-selectin–dependent CHO-E cell rolling. (B) KG1a membrane proteins (10 μg) were treated with _N_-glycosidase F, separated on a reducing 6% SDS-PAGE gel, and immunostained with HECA-452. (C) Immunoprecipitated PSGL-1 was resolved on a reducing 6% SDS-PAGE gel and Western blotted with either HECA-452 (left) or anti–PSGL-1 antibody 4H10 (right). (lane 1) 10 μg of total KG1a membrane protein; (lane 2) immunoprecipitated PSGL-1 from 100 μg of KG1a membrane protein; (lane 3) 100 μg of total KG1a membrane protein, and (lane 4) immunoprecipitated PSGL-1, from 100 μg of KG1a membrane protein. Note that HECA-452–stained bands at 140 and 220 kD correspond to PSGL-1. (D) Isotype control or Hermes-1 immunoprecipitated CD44 from KG1a membrane protein (50 μg) was resolved on a reducing 9% SDS-PAGE gel and immunoblotted with HECA-452. Immunoprecipitated CD44 from KG1a membrane proteins (50 μg) treated with _N_-glycosidase F was also immunoblotted with HECA-452. Though CHO-E cell rolling frequencies are presented as the mean ± SD of E-selectin–mediated cell rolling at 3.8 dynes/cm2 measured on the 100-kD isoform of CD44, no CHO-E rolling was observed along the entire length of the _N_-glycosidase F–treated lane.
Figure 1
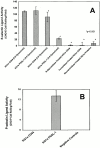
Expression of HECA-452–reactive glycoproteins on human hematopoietic cells and E-selectin ligand activity of human hematopoietic cell lines. (A) Membrane preparations of human hematopoietic cell lines, KG1a (10 μg), HL60 (100 μg), RPMI-8402 (100 μg), and K562 (100 μg) were resolved on a reducing 6% SDS-PAGE gel and immunoblotted with HECA-452. (B) Parallel plate flow chamber analysis of CHO-E cell tethering and rolling on glutaraldehyde-fixed monolayers of hematopoietic cell lines (shear stress of 2.8 dynes/cm2). Twofold more CHO-E cell tethering and rolling was observed on KG1a than on HL60 cell monolayers, which was not affected by OSGE pretreatment. There was no CHO-E cell rolling on RPMI-8402 or K562 cell monolayers. Negative controls consisted of CHO-mock transfectants and CHO-E cells treated with anti–E-selectin Abs (10 μg/ml). Data are presented as mean ± SD CHO-E cell rolling per field × 5 fields, minimum of three experiments.
Figure 2
Human hematopoietic cell rolling on freshly isolated human BMEC. KG1a, HL60, RPMI-8402, and K562 were perfused over live IL-1α–treated primary BMEC cultures at 2.8 dynes/cm2 in the parallel plate flow chamber, and cell rolling was observed and recorded for video analysis. Controls consisted of untreated BMEC and IL-1α–treated BMEC in the presence of anti–E-selectin mAb. Untreated BMEC showed no E-selectin ligand activity, and rolling on IL-1α–treated BMEC was eliminated by incubation with function-blocking anti–E-selectin mAb 68-5H11. Data represent mean ± SD cell rolling frequency per 100 × magnified field × 5 fields of view, minimum of three experiments. Note that KG1a cell rolling was 3.5-fold greater than that of HL60 cells.
Figure 4
Exhaustive immunoprecipitation of CD44 (Hermes-1) and blot rolling assay of residual E-selectin ligand activity. Hermes-1 immunoblot (A) or HECA-452 immunoblot ( B) of KG1a lysate (10 μg) subjected to three rounds of immunoprecipitation with Hermes-1 mAb. (Pre-Ippt) Total KG1a lysate 10 μg; (lane 1) first round Hermes-1 immunoprecipitate; (lane 2) second round Hermes-1 immunoprecipitate; (lane 3) third round Hermes-1 immunoprecipitate; (lane 4) residual lysate after three rounds of Hermes-1 immunoprecipitation. E-selectin ligand activity (CHO-E cell rolling) correlates with intensity of the Hermes-1 staining and of HECA-452 staining of 100-kD band, is reduced after each round of Hermes-1 immunoprecipitation (A), and was completely absent in the residual lysate material after the third round of immunoprecipitation (lane 4, A and B). CHO-E cell rolling was also eliminated over the 190-kD band by exhaustive Hermes-1 immunoprecipitation, whereas CHO-E cell rolling on 120-kD stained band was markedly reduced, and the 140-kD band (PSGL-1) retained full activity (B, lane 4).
Figure 6
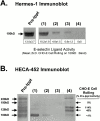
HECA-452–reactive CD44 from freshly isolated normal human HPCs and from human leukemic blasts functions as an E-selectin ligand. (Panel A) HECA-452 staining of CD44 immunoprecipitated from (a) human BM mononuclear cells (108 cells), (b) CD34−/lineage+ cells (107 cells), (c) CD34+/lineage− cells (107 cells), (d) CD34−/lineage+ cells (108 cells). HECA-452 staining of CD44 and CHO-E cell rolling was observable only on the 100-kD CD44 immunoprecipitated from CD34+/lineage− cells (data are mean ± SD cell rolling/field on the 100-kD band). (B) Membrane proteins (50 μg) isolated from circulating blasts from an AML (M5) were resolved on a 9% SDS-PAGE gel and immunoblotted with HECA-452. Treatment of membrane proteins with _N_-glycosidase F markedly diminished HECA-452 staining. (C) Blot rolling assay results of AML (M5) membrane protein (50 μg) immunoprecipitated with isotype control or with Hermes-1 mAb, and of _N_-glycosidase F–treated Hermes-1 immunoprecipitates. Though data shown in parentheses is CHO-E cell rolling over the 100-kD band, no rolling was observed over the entire length of the lane corresponding to _N_-glycosidase F–treated protein. (D) Immunoprecipitated CD44 from membrane preparations (50 μg) of an AML (M0), AML (M1) and atypical CML (brc/abl−), and of human BMEC line (BMEC-1; 100 μg total protein), was separated on a 6% SDS-PAGE gel, immunostained with HECA-452, and evaluated for E-selectin ligand activity. E-selectin ligand activity correlates with intensity of HECA-452 staining of 100-kD band. (E) Western blot of immunoprecipitated CD44 from BMEC-1 stained with Hermes-1 mAb.
Figure 6
HECA-452–reactive CD44 from freshly isolated normal human HPCs and from human leukemic blasts functions as an E-selectin ligand. (Panel A) HECA-452 staining of CD44 immunoprecipitated from (a) human BM mononuclear cells (108 cells), (b) CD34−/lineage+ cells (107 cells), (c) CD34+/lineage− cells (107 cells), (d) CD34−/lineage+ cells (108 cells). HECA-452 staining of CD44 and CHO-E cell rolling was observable only on the 100-kD CD44 immunoprecipitated from CD34+/lineage− cells (data are mean ± SD cell rolling/field on the 100-kD band). (B) Membrane proteins (50 μg) isolated from circulating blasts from an AML (M5) were resolved on a 9% SDS-PAGE gel and immunoblotted with HECA-452. Treatment of membrane proteins with _N_-glycosidase F markedly diminished HECA-452 staining. (C) Blot rolling assay results of AML (M5) membrane protein (50 μg) immunoprecipitated with isotype control or with Hermes-1 mAb, and of _N_-glycosidase F–treated Hermes-1 immunoprecipitates. Though data shown in parentheses is CHO-E cell rolling over the 100-kD band, no rolling was observed over the entire length of the lane corresponding to _N_-glycosidase F–treated protein. (D) Immunoprecipitated CD44 from membrane preparations (50 μg) of an AML (M0), AML (M1) and atypical CML (brc/abl−), and of human BMEC line (BMEC-1; 100 μg total protein), was separated on a 6% SDS-PAGE gel, immunostained with HECA-452, and evaluated for E-selectin ligand activity. E-selectin ligand activity correlates with intensity of HECA-452 staining of 100-kD band. (E) Western blot of immunoprecipitated CD44 from BMEC-1 stained with Hermes-1 mAb.
Figure 6
HECA-452–reactive CD44 from freshly isolated normal human HPCs and from human leukemic blasts functions as an E-selectin ligand. (Panel A) HECA-452 staining of CD44 immunoprecipitated from (a) human BM mononuclear cells (108 cells), (b) CD34−/lineage+ cells (107 cells), (c) CD34+/lineage− cells (107 cells), (d) CD34−/lineage+ cells (108 cells). HECA-452 staining of CD44 and CHO-E cell rolling was observable only on the 100-kD CD44 immunoprecipitated from CD34+/lineage− cells (data are mean ± SD cell rolling/field on the 100-kD band). (B) Membrane proteins (50 μg) isolated from circulating blasts from an AML (M5) were resolved on a 9% SDS-PAGE gel and immunoblotted with HECA-452. Treatment of membrane proteins with _N_-glycosidase F markedly diminished HECA-452 staining. (C) Blot rolling assay results of AML (M5) membrane protein (50 μg) immunoprecipitated with isotype control or with Hermes-1 mAb, and of _N_-glycosidase F–treated Hermes-1 immunoprecipitates. Though data shown in parentheses is CHO-E cell rolling over the 100-kD band, no rolling was observed over the entire length of the lane corresponding to _N_-glycosidase F–treated protein. (D) Immunoprecipitated CD44 from membrane preparations (50 μg) of an AML (M0), AML (M1) and atypical CML (brc/abl−), and of human BMEC line (BMEC-1; 100 μg total protein), was separated on a 6% SDS-PAGE gel, immunostained with HECA-452, and evaluated for E-selectin ligand activity. E-selectin ligand activity correlates with intensity of HECA-452 staining of 100-kD band. (E) Western blot of immunoprecipitated CD44 from BMEC-1 stained with Hermes-1 mAb.
Figure 5
HECA-452–reactive CD44 is a more avid E-Selectin ligand than PSGL-1. Equivalent amounts (1 μg) of either immunoprecipitated CD44 or PSGL-1 were analyzed for E-selectin and P-selectin ligand activity in the parallel plate flow chamber. (A) E-selectin–mediated CHO-E cell rolling was observed at 2.8 dynes/cm2 on KG1a CD44, but was significantly lower on KG1a PSGL-1 at 2.8 dynes/cm2 (P < 0.001). _N_-glycosidase F- and α-L-fucosidase-treated KG1a CD44, and Vibrio cholerae neuraminidase treatment of KG1a membrane protein abrogated CHO-E cell rolling (P < 0.001), and no CHO-E cell rolling was observed on isotype control rat IgG– or mouse IgG–immunoprecipitated KG1a protein (data not shown). (B) CHO-P cell rolling was observed on KG1a PSGL-1 but not on KG1a CD44 (2.8 dyn/cm2). No rolling was observed on negative controls (CHO-Mock cells and CHO-P cells pretreated with function-blocking anti–P-selectin mAb AK-4 [10 μg/ml]).
Similar articles
- differential L-selectin binding activities of human hematopoietic cell L-selectin ligands, HCELL and PSGL-1.
Dimitroff CJ, Lee JY, Schor KS, Sandmaier BM, Sackstein R. Dimitroff CJ, et al. J Biol Chem. 2001 Dec 14;276(50):47623-31. doi: 10.1074/jbc.M105997200. Epub 2001 Oct 8. J Biol Chem. 2001. PMID: 11591704 - PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin.
Katayama Y, Hidalgo A, Furie BC, Vestweber D, Furie B, Frenette PS. Katayama Y, et al. Blood. 2003 Sep 15;102(6):2060-7. doi: 10.1182/blood-2003-04-1212. Epub 2003 May 22. Blood. 2003. PMID: 12763924 - Analysis of glycoprotein E-selectin ligands on human and mouse marrow cells enriched for hematopoietic stem/progenitor cells.
Merzaban JS, Burdick MM, Gadhoum SZ, Dagia NM, Chu JT, Fuhlbrigge RC, Sackstein R. Merzaban JS, et al. Blood. 2011 Aug 18;118(7):1774-83. doi: 10.1182/blood-2010-11-320705. Epub 2011 Jun 9. Blood. 2011. PMID: 21659548 Free PMC article. - The biology of CD44 and HCELL in hematopoiesis: the 'step 2-bypass pathway' and other emerging perspectives.
Sackstein R. Sackstein R. Curr Opin Hematol. 2011 Jul;18(4):239-48. doi: 10.1097/MOH.0b013e3283476140. Curr Opin Hematol. 2011. PMID: 21546828 Free PMC article. Review. - Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow.
Zarbock A, Ley K, McEver RP, Hidalgo A. Zarbock A, et al. Blood. 2011 Dec 22;118(26):6743-51. doi: 10.1182/blood-2011-07-343566. Epub 2011 Oct 20. Blood. 2011. PMID: 22021370 Free PMC article. Review.
Cited by
- Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance.
Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, Lévesque JP. Winkler IG, et al. Nat Med. 2012 Nov;18(11):1651-7. doi: 10.1038/nm.2969. Epub 2012 Oct 21. Nat Med. 2012. PMID: 23086476 - Lectin chromatography/mass spectrometry discovery workflow identifies putative biomarkers of aggressive breast cancers.
Drake PM, Schilling B, Niles RK, Prakobphol A, Li B, Jung K, Cho W, Braten M, Inerowicz HD, Williams K, Albertolle M, Held JM, Iacovides D, Sorensen DJ, Griffith OL, Johansen E, Zawadzka AM, Cusack MP, Allen S, Gormley M, Hall SC, Witkowska HE, Gray JW, Regnier F, Gibson BW, Fisher SJ. Drake PM, et al. J Proteome Res. 2012 Apr 6;11(4):2508-20. doi: 10.1021/pr201206w. Epub 2012 Mar 13. J Proteome Res. 2012. PMID: 22309216 Free PMC article. - Hematopoietic progenitor cells (HPC) from mobilized peripheral blood display enhanced migration and marrow homing compared to steady-state bone marrow HPC.
Bonig H, Priestley GV, Oehler V, Papayannopoulou T. Bonig H, et al. Exp Hematol. 2007 Feb;35(2):326-34. doi: 10.1016/j.exphem.2006.09.017. Exp Hematol. 2007. PMID: 17258081 Free PMC article. - Coordinated and unique functions of the E-selectin ligand ESL-1 during inflammatory and hematopoietic recruitment in mice.
Sreeramkumar V, Leiva M, Stadtmann A, Pitaval C, Ortega-Rodríguez I, Wild MK, Lee B, Zarbock A, Hidalgo A. Sreeramkumar V, et al. Blood. 2013 Dec 5;122(24):3993-4001. doi: 10.1182/blood-2013-07-514497. Epub 2013 Oct 8. Blood. 2013. PMID: 24106206 Free PMC article. - The Bone Marrow Niche - The Tumor Microenvironment That Ensures Leukemia Progression.
Cardoso BA. Cardoso BA. Adv Exp Med Biol. 2020;1219:259-293. doi: 10.1007/978-3-030-34025-4_14. Adv Exp Med Biol. 2020. PMID: 32130704 Review.
References
- Asa D., Raycroft L., Ma L. The P-selectin glycoprotein ligand functions as a common human leukocyte ligand for P- and E-selectins. J. Biol. Chem. 1995;270:11662–11670. - PubMed
- Aruffo A., Stamenkovic I., Melnick M., Underhill C.B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. - PubMed
- Candal F.J., Rafii S., Parker J.T., Ades E.W., Ferris B., Nachman R.L., Kellar K.L. BMEC-1a human bone marrow microvascular endothelial cell line with primary cell characteristics. Microvasc. Res. 1996;52:221–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous