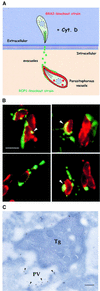
Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole - PubMed (original) (raw)
Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole
S Håkansson et al. EMBO J. 2001.
Abstract
Rapid discharge of secretory organelles called rhoptries is tightly coupled with host cell entry by the protozoan parasite Toxoplasma gondii. Rhoptry contents were deposited in clusters of vesicles within the host cell cytosol and within the parasitophorous vacuole. To examine the fate of these rhoptry-derived secretory vesicles, we utilized cytochalasin D to prevent invasion, leading to accumulation of protein-rich vesicles in the host cell cytosol. These vesicles lack an internal parasite and are hence termed evacuoles. Like the mature parasite-containing vacuole, evacuoles became intimately associated with host cell mitochondria and endoplasmic reticulum, while remaining completely resistant to fusion with host cell endosomes and lysosomes. In contrast, evacuoles were recruited to pre-existing, parasite-containing vacuoles and were capable of fusing and delivering their contents to these compartments. Our findings indicate that a two-step process involving direct rhoptry secretion into the host cell cytoplasm followed by incorporation into the vacuole generates the parasitophorous vacuole occupied by TOXOPLASMA: The characteristic properties of the mature vacuole are likely to be determined by this early delivery of rhoptry components.
Figures
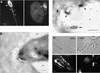
Fig. 1. Distribution of rhoptry-derived secretory vesicles formed by Toxoplasma in HFF cells. (A) IF staining of rhoptry proteins discharged during normal invasion. Release of rhoptry proteins into the parasitophorous vacuole resulted in staining of the vacuolar membrane (arrows) and clusters of small vesicles within the host cell cytosol (arrowheads). Cells were fixed, permeabilized and stained with mAb Tg49 anti-ROP1 followed by fluorescently conjugated goat anti-mouse IgG. Counter-staining with DAPI reveals the host cell nucleus (HN) in proximity to the parasitophorous vacuole. Scale bar = 5 µm. (B) CryoimmunoEM of rhoptry-derived secretory vesicles formed during normal invasion. Secretory vesicles that contain ROP proteins (*) lie adjacent to the parasitophorous vacuole (PV) but are outside the limiting membrane of this compartment (arrows). Stained with mAb Tg49 to ROP1 followed by goat anti-mouse IgG conjugated to 18 nm gold. A partially discharged rhoptry (R), which contains ROP1, is located near the anterior end of the parasite (Tg). Scale bar = 250 nm. (C) Higher magnification of a ROP2-positive satellite vesicle formed in the vicinity of a parasite-containing vacuole (not shown) during invasion. The limiting membrane of the rhoptry-derived secretory vacuole is not readily apparent, yet it contains internal membranous structures (arrows). The lumen of the secretory vesicle is marked by *. ROP2 was visualized by staining with rabbit anti-ROP2 followed by goat anti-rabbit IgG conjugated to 18 nm gold. Scale bar = 250 nm. (D) IF of rhoptry-derived secretory vesicles formed under CytD block. ROP1-positive vesicles were formed within the host cell cytosol, emanating from the site of parasite attachment (arrowheads). In the absence of nocodazole (–NOC), migration away from the site of injection led to ribbons of vesicles stretched out within the host cell cytosol. In the presence of NOC, migration was blocked and vesicles remain concentrated at the site of injection (arrowhead in lower right panel). Cells were fixed, permeabilized and stained for the parasite protein ROP1 using mAb Tg49 followed by BODIPY-conjugated goat anti-mouse IgG. Scale bar = 5 µm.
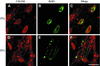
Fig. 2. Confocal micrographs showing uptake of the lipophilic dye CM-DiIC16 from the host cell plasma membrane into parasitophorous vacuoles (PVs) (top panels) but not into evacuoles (EVs) (bottom panels). Pre-labeled HFF monolayers were challenged with parasites to allow formation of parasite-containing vacuoles (A–C) or challenged with parasites in the presence of 1 µM CytD to form evacuoles (D–F). Monolayers were fixed, permeabilized and stained for the parasite rhoptry protein ROP1 using mAb Tg49 followed by BODIPY-conjugated goat anti-mouse IgG to visualize either parasitophorous vacuoles (B) or evacuoles (E). Scale bar = 10 µm.
Fig. 3. Confocal immunofluorescence micrographs showing the absence of fusion of early and late endocytic vesicles with evacuoles. Mouse 3T3 cells containing evacuoles (A–C) were fixed after 15 min, permeabilized and stained with anti-TfR mAb R17.217.13 followed by FITC-conjugated goat anti-rat IgG. HFF cells containing evacuoles (D–F) were fixed after 60 min, permeabilized and incubated with anti-LAMP1 mAb H4A3 followed by Texas Red-conjugated goat anti-mouse IgG. Evacuoles were visualized by staining for the parasite rhoptry protein ROP2 using a specific rabbit serum followed by Texas Red (B)- or BODIPY (E)-conjugated goat anti-rabbit IgG. Scale bar = 10 µm.
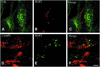
Fig. 4. Confocal immunofluorescence micrographs showing association of host cell mitochondria with parasitophorous vacuoles (PVs) and evacuoles (EVs). HFF cells were labeled with the mitochondrial vital dye MitoTracker Red CMXRos and challenged with parasites to form parasitophorous vacuoles in the absence of drug (A–C) or challenged with parasites in the presence of 1 µM CytD to form evacuoles (D–F). Following a 45 min chase, monolayers were fixed, permeabilized and stained for the parasite rhoptry protein ROP2 using a specific rabbit serum followed by BODIPY-conjugated goat anti-rabbit IgG to visualize the parasitophorous vacuoles (B) or evacuoles (E). Scale bars = 10 µm.
Fig. 5. CryoimmunoEM localization of rhoptry proteins within evacuoles formed in host cells. (A) Immunogold staining for the parasite rhoptry protein ROP1 (detected with mAb Tg49 followed by goat anti-mouse IgG conjugated to 18 nm gold). A large cluster of evacuoles (EV), which contain internal electron-dense profiles (arrows), is closely associated with host cell mitochondria (M). Insert shows an enlarged region containing a multilamellar structure. (B) Immunogold staining for the parasite rhoptry protein ROP2 (detected with rabbit anti-ROP2 followed by goat anti-rabbit IgG conjugated to 12 nm gold) reveals smaller clusters of evacuoles (EV) in close association with mitochondria (M). (C) Evacuoles often contained electron-dense membranous structures protruding into the lumen. Examples from samples stained with rabbit anti-ROP2 followed by goat anti-rabbit IgG conjugated to 18 nm gold. Scale bars = 250 µm.
Fig. 6. CryoimmunoEM of evacuoles associated with host ER membranes. (A) Immunogold staining for the parasite rhoptry protein ROP2 (detected with rabbit anti-ROP2 followed by goat anti-rabbit IgG conjugated to 12 nm gold) reveals an evacuole wrapped with a double layer of host cell membranes that resembles ER (arrowheads). M, mitochondria. (B) Colocalization of ROP2 (detected with rabbit anti-ROP2 followed by goat anti-rabbit IgG conjugated to 18 nm gold) with the host ER marker, PDI (detected with mAb RL77 followed by goat anti-mouse IgG conjugated to 12 nm gold). Host membranes that stain positively for PDI (arrowheads) surround a prominent evacuole (EV). Scale bars = 250 nm. (C and D) Examples of evacuoles that were closely associated with ER membranes as shown by colocalization of ROP2 (18 nm gold) and PDI (12 nm gold). Staining as in (B). Scale bars = 250 nm.
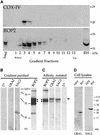
Fig. 7. Western blot analyses demonstrating co-isolation of evacuoles with host cell mitochondria. (A) Host cell mitochondria and evacuoles cosedimented on density gradients. The distribution of the host mitochondria and evacuoles along the gradient was established by probing replicate blots with mAb 1A12-A12 against the mitochondrial marker COX-IV (top) or with rabbit polyclonal antibody against the parasite rhoptry protein ROP2 (bottom) followed by the appropriate HRP-conjugated secondary antibodies. (B) Immunoblot analysis of parasite proteins that cofractionate with host cell mitochondria. Proteins in the combined fractions 3 and 4 from (A) were resolved by SDS–PAGE, blotted onto nitrocellulose and probed with rabbit polyclonal antibodies followed by HRP-conjugated goat anti-rabbit IgG. Evacuoles co-sedimented with mitochondria, as shown by the presence of the parasite rhoptry protein ROP2. A small amount of the parasite surface protein SAG1 was detected while GRA_2_ was absent; (2°) secondary antibody alone. (C) Evacuoles physically associated with host cell mitochondria. Mitochondria were affinity isolated from the gradient fractions shown in (B) using mAb II-14-10 against the host cell mitochondrial protein prohibitin and subjected to SDS–PAGE and immunoblot analysis as described above. The parasite rhoptry protein ROP2 was specifically pulled down with mitochondria, while the parasite cell surface protein SAG1, also found in the starting fraction, was not. Arrowheads indicate the position of ROP2 (the lower bands represent typical degradation products); the arrow to the right identifies a non-specific, cross-reacting protein recognized by the rabbit sera. The relative migrations of molecular weight standards are indicated in kDa. (D) Control western blots showing the relative migration of GRA2 (28 kDa) and SAG1 (36 kDa when reduced). HFF, lysates of host cells; RH, parasite lysate. Probed with mono-specific, rabbit polyclonal sera followed by HRP-conjugated secondary goat anti-rabbit IgG.
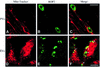
Fig. 8. Evacuoles associate and fuse with parasitophorous vacuoles. (A) Model depicting the double mutant strategy used to track the association of evacuoles with parasitophorous vacuoles. HFF cell monolayers were challenged with a rop1 knockout strain to form parasitophorous vacuoles, rinsed thoroughly and challenged anew with a gra2 knockout strain in the presence of 1 µM CytD to form evacuoles. Parasite-containing vacuoles that are formed by the first wave of parasites are Rop1–/Gra2+. In contrast, only evacuoles formed by the second wave of parasites are Rop1+. (B) Examples of evacuoles that formed tight associations with pre-existing parasitophorous vacuoles. Evacuoles often enveloped portions of the parasitophorous vacuole and formed ribbons along the surface of the parasite-containing vacuole. Prominent colocalization was detected as a yellow fluorescence signal (arrowheads). Doubly challenged monolayers were fixed, permeabilized and stained for ROP1 using the mAb Tg49 followed by BODIPY-conjugated goat anti-mouse IgG and rabbit anti-GRA2 followed by Texas Red-conjugated goat anti-rabbit IgG. Scale bar = 10 µm. (C) Doubly challenged HFF cell monolayers processed for cryoimmunoEM. Fusion of evacuoles with pre-existing parasitophorous vacuoles (PV) resulted in colocalization of ROP1 (18 nm gold) and GRA2 (12 nm gold) in the same vacuole (arrowheads). Evacuoles were visualized by staining for ROP1 using mAb Tg49 followed by goat anti-mouse IgG conjugated to 18 nm gold. Parasite-containing vacuoles were detected by staining with rabbit anti-GRA2 followed by goat anti-rabbit conjugated to 12 nm gold. Tg denotes Toxoplasma cell. Scale bar = 250 nm.
Similar articles
- The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm.
Beckers CJ, Dubremetz JF, Mercereau-Puijalon O, Joiner KA. Beckers CJ, et al. J Cell Biol. 1994 Nov;127(4):947-61. doi: 10.1083/jcb.127.4.947. J Cell Biol. 1994. PMID: 7962077 Free PMC article. - A host cell membrane microdomain is a critical factor for organelle discharge by Toxoplasma gondii.
Tahara M, Andrabi SB, Matsubara R, Aonuma H, Nagamune K. Tahara M, et al. Parasitol Int. 2016 Oct;65(5 Pt A):378-88. doi: 10.1016/j.parint.2016.05.012. Epub 2016 May 20. Parasitol Int. 2016. PMID: 27217289 - Interrelations between the parasitophorous vacuole of Toxoplasma gondii and host cell organelles.
Magno RC, Straker LC, de Souza W, Attias M. Magno RC, et al. Microsc Microanal. 2005 Apr;11(2):166-74. doi: 10.1017/S1431927605050129. Microsc Microanal. 2005. PMID: 15817146 - Biogenesis of and activities at the Toxoplasma gondii parasitophorous vacuole membrane.
Sinai AP. Sinai AP. Subcell Biochem. 2008;47:155-64. doi: 10.1007/978-0-387-78267-6_12. Subcell Biochem. 2008. PMID: 18512349 Review. - Rhoptries are major players in Toxoplasma gondii invasion and host cell interaction.
Dubremetz JF. Dubremetz JF. Cell Microbiol. 2007 Apr;9(4):841-8. doi: 10.1111/j.1462-5822.2007.00909.x. Epub 2007 Mar 8. Cell Microbiol. 2007. PMID: 17346309 Review.
Cited by
- The secreted kinase ROP17 promotes Toxoplasma gondii dissemination by hijacking monocyte tissue migration.
Drewry LL, Jones NG, Wang Q, Onken MD, Miller MJ, Sibley LD. Drewry LL, et al. Nat Microbiol. 2019 Nov;4(11):1951-1963. doi: 10.1038/s41564-019-0504-8. Epub 2019 Jul 22. Nat Microbiol. 2019. PMID: 31332383 Free PMC article. - A highly sensitive FRET-based approach reveals secretion of the actin-binding protein toxofilin during Toxoplasma gondii infection.
Lodoen MB, Gerke C, Boothroyd JC. Lodoen MB, et al. Cell Microbiol. 2010 Jan;12(1):55-66. doi: 10.1111/j.1462-5822.2009.01378.x. Epub 2009 Sep 2. Cell Microbiol. 2010. PMID: 19732057 Free PMC article. - 4-Bromophenacyl bromide specifically inhibits rhoptry secretion during Toxoplasma invasion.
Ravindran S, Lodoen MB, Verhelst SH, Bogyo M, Boothroyd JC. Ravindran S, et al. PLoS One. 2009 Dec 2;4(12):e8143. doi: 10.1371/journal.pone.0008143. PLoS One. 2009. PMID: 19956582 Free PMC article. - Functional Characterization of the Thrombospondin-Related Paralogous Proteins Rhoptry Discharge Factors 1 and 2 Unveils Phenotypic Plasticity in Toxoplasma gondii Rhoptry Exocytosis.
Possenti A, Di Cristina M, Nicastro C, Lunghi M, Messina V, Piro F, Tramontana L, Cherchi S, Falchi M, Bertuccini L, Spano F. Possenti A, et al. Front Microbiol. 2022 Jun 9;13:899243. doi: 10.3389/fmicb.2022.899243. eCollection 2022. Front Microbiol. 2022. PMID: 35756016 Free PMC article. - Cell invasion and strain dependent induction of suppressor of cytokine signaling-1 by Toxoplasma gondii.
Stutz A, Kessler H, Kaschel ME, Meissner M, Dalpke AH. Stutz A, et al. Immunobiology. 2012 Jan;217(1):28-36. doi: 10.1016/j.imbio.2011.08.008. Epub 2011 Aug 27. Immunobiology. 2012. PMID: 22015046 Free PMC article.
References
- Bannister L.H., Mitchell,G.H., Butcher,G.A. and Dennis,E.D. (1986) Lamellar membranes associated with rhoptries in erythrocytic merozoites of Plasmodium knowlesi: a clue to the mechanism of invasion. Parasitology, 92, 291–303. - PubMed
- Beckers C.J.M., Dubremetz,J.F., Mercereau-Puijalon,O. and Joiner,K.A. (1994) The Toxoplasma gondii rhoptry protein ROP2 is inserted into the parasitophorous vacuole membrane, surrounding the intracelluar parasite, and is exposed to the host cell cytoplasm. J. Cell Biol., 127, 947–961. - PMC - PubMed
- Brecht S., Carruthers,V.B., Ferguson,D.J., Giddings,O.K., Wang,G., Jaekle,U., Harper,J.M., Sibley,L.D. and Soldati,D. (2001) The Toxoplasma micronemal protein MIC4 is an adhesin composed of six conserved apple domains. J. Biol. Chem., 276, 4119–4127. - PubMed
- Carruthers V.B. and Sibley,L.D. (1997) Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol., 73, 114–123. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources