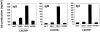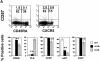Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells - PubMed (original) (raw)
Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells
C H Kim et al. J Exp Med. 2001.
Abstract
The T helper (Th) cell pool is composed of specialized cells with heterogeneous effector functions. Apart from Th1 and 2 cells, CXCR5+ T cells have been suggested to be another type of effector T cell specialized for B cell help. We show here that CXCR5+ T cells are heterogeneous, and we identify subsets of CXCR5+ CD4 T cells that differ in function and microenvironmental localization in secondary lymphoid tissues. CD57+CXCR5 T cells, hereafter termed germinal center Th (GC-Th) cells, are localized only in GCs, lack CCR7, and are highly responsive to the follicular chemokine B lymphocyte chemoattractant but not to the T cell zone EBI1-ligand chemokine. Importantly, GC-Th cells are much more efficient than CD57-CXCR5+ T cells or CXCR5- T cells in inducing antibody production from B cells. Consistent with their function, GC-Th cells produce elevated levels of interleukin 10 upon stimulation which, with other cytokines and costimulatory molecules, may help confer their B cell helper activity. Our results demonstrate that CXCR5+ T cells are functionally heterogeneous and that the GC-Th cells, a small subset of CXCR5+ T cells, are the key helpers for B cell differentiation and antibody production in lymphoid tissues.
Figures
Figure 1
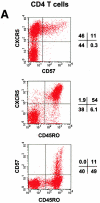
Identification of CD57+ CXCR5+ T cells and their localization in GCs. (A) Flow analyses of CD57 and CXCR5 expression on tonsil CD4 T cells. (B) Specific localization of CD57+ CD4 T cells in GCs. (C) Localization of CD57+ CXCR5+ CD4 T cells in GCs. Antibodies to CD57 (green), CD4 (blue), and IgD (red; B) or CXCR5 (red; C) were used for in situ immunohistochemistry.
Figure 1
Identification of CD57+ CXCR5+ T cells and their localization in GCs. (A) Flow analyses of CD57 and CXCR5 expression on tonsil CD4 T cells. (B) Specific localization of CD57+ CD4 T cells in GCs. (C) Localization of CD57+ CXCR5+ CD4 T cells in GCs. Antibodies to CD57 (green), CD4 (blue), and IgD (red; B) or CXCR5 (red; C) were used for in situ immunohistochemistry.
Figure 2
Chemotactic responses (A) and chemokine receptor expression (B) by CD57+/2CXCR5+/− CD4 T cells. Optimal concentrations of 5 μg/ml BLC, 1 μg/ml ELC, and 100 ng/ml SDF-1 were used for chemotaxis experiments. Freshly isolated tonsil cells were used for chemotaxis and flow analyses of tonsil CD4 T cell subsets. Representatives of three independent experiments are shown.
Figure 2
Chemotactic responses (A) and chemokine receptor expression (B) by CD57+/2CXCR5+/− CD4 T cells. Optimal concentrations of 5 μg/ml BLC, 1 μg/ml ELC, and 100 ng/ml SDF-1 were used for chemotaxis experiments. Freshly isolated tonsil cells were used for chemotaxis and flow analyses of tonsil CD4 T cell subsets. Representatives of three independent experiments are shown.
Figure 3
Effector machinery of CD57+/−CD45RA+/− or CD57+/− CXCR5+/− CD4 T cells. (A) Intracellular cytokine analyses of TNF-α, IL-2, IL-10, IFN-γ, and IL-4. (B) ELISA of IL-10 and IL-4. Isolated T cells were activated with 50 ng/ml PMA and 1 μg/ml ionomycin for 4 h for intracellular cytokine analyses, or 24 h for ELISA of IL-10 and IL-4. Representatives of at least four independent experiments are shown.
Figure 3
Effector machinery of CD57+/−CD45RA+/− or CD57+/− CXCR5+/− CD4 T cells. (A) Intracellular cytokine analyses of TNF-α, IL-2, IL-10, IFN-γ, and IL-4. (B) ELISA of IL-10 and IL-4. Isolated T cells were activated with 50 ng/ml PMA and 1 μg/ml ionomycin for 4 h for intracellular cytokine analyses, or 24 h for ELISA of IL-10 and IL-4. Representatives of at least four independent experiments are shown.
Figure 4
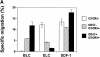
Spontaneous B cell helper activity of CD57+/− CXCR5+/− CD4 T cells. Sorted CD57+CXCR5+, CD57− CXCR5+, and CXCR5− CD4 T cells were cocultured with B cells from the same tonsil for 11–13 d in the absence of any stimulatory agents followed by analyses of secreted IgG, IgA, and IgM. Representatives of at least three independent experiments are shown.
Figure 5
Phenotype and effector function of circulating CXCR5+ T cells. (A) Surface phenotype of circulating naive or CXCR5+/− memory T cells. **Significant differences between CXCR5+ and CXCR5− memory cells. (B) IL-4/IFN-γ production capabilities of CXCR5+/− T cells during repeated T cell receptor activation (each cycle is composed of 4-d activation with anti-CD3 and anti-CD28 followed by 3-d resting in the presence of IL-2). (C) B cell help activity of circulating CXCR5+ T cells after T cell receptor activation. Naive (IgD+) or memory (IgD−) B cells (2 × 104) from peripheral blood were cultured for 14 d in the presence or absence of various numbers (103, 5 × 103, 104, 2.5 × 104, 5 × 104, and 105) of autologous T cells (CXCR5−CD45RA+, CXCR5+CD45RA−, or CXCR5− CD45RA−). Concentrations of IgG, IgA, and IgM in the culture supernatants were measured by ELISA. T cell numbers required for peak levels of antibody production varied among donors or experiments. One peak value with the best antibody production in each T cell group is shown. (D) Loss of CXCR5 expression during T cell receptor activation with anti-CD3 and anti-CD28. Error bars indicate SD of results from at least five different experiments (A). Representatives of three independent experiments are shown (B and D), and results from seven different donors (C) are shown.
Figure 5
Phenotype and effector function of circulating CXCR5+ T cells. (A) Surface phenotype of circulating naive or CXCR5+/− memory T cells. **Significant differences between CXCR5+ and CXCR5− memory cells. (B) IL-4/IFN-γ production capabilities of CXCR5+/− T cells during repeated T cell receptor activation (each cycle is composed of 4-d activation with anti-CD3 and anti-CD28 followed by 3-d resting in the presence of IL-2). (C) B cell help activity of circulating CXCR5+ T cells after T cell receptor activation. Naive (IgD+) or memory (IgD−) B cells (2 × 104) from peripheral blood were cultured for 14 d in the presence or absence of various numbers (103, 5 × 103, 104, 2.5 × 104, 5 × 104, and 105) of autologous T cells (CXCR5−CD45RA+, CXCR5+CD45RA−, or CXCR5− CD45RA−). Concentrations of IgG, IgA, and IgM in the culture supernatants were measured by ELISA. T cell numbers required for peak levels of antibody production varied among donors or experiments. One peak value with the best antibody production in each T cell group is shown. (D) Loss of CXCR5 expression during T cell receptor activation with anti-CD3 and anti-CD28. Error bars indicate SD of results from at least five different experiments (A). Representatives of three independent experiments are shown (B and D), and results from seven different donors (C) are shown.
Figure 5
Phenotype and effector function of circulating CXCR5+ T cells. (A) Surface phenotype of circulating naive or CXCR5+/− memory T cells. **Significant differences between CXCR5+ and CXCR5− memory cells. (B) IL-4/IFN-γ production capabilities of CXCR5+/− T cells during repeated T cell receptor activation (each cycle is composed of 4-d activation with anti-CD3 and anti-CD28 followed by 3-d resting in the presence of IL-2). (C) B cell help activity of circulating CXCR5+ T cells after T cell receptor activation. Naive (IgD+) or memory (IgD−) B cells (2 × 104) from peripheral blood were cultured for 14 d in the presence or absence of various numbers (103, 5 × 103, 104, 2.5 × 104, 5 × 104, and 105) of autologous T cells (CXCR5−CD45RA+, CXCR5+CD45RA−, or CXCR5− CD45RA−). Concentrations of IgG, IgA, and IgM in the culture supernatants were measured by ELISA. T cell numbers required for peak levels of antibody production varied among donors or experiments. One peak value with the best antibody production in each T cell group is shown. (D) Loss of CXCR5 expression during T cell receptor activation with anti-CD3 and anti-CD28. Error bars indicate SD of results from at least five different experiments (A). Representatives of three independent experiments are shown (B and D), and results from seven different donors (C) are shown.
Figure 5
Phenotype and effector function of circulating CXCR5+ T cells. (A) Surface phenotype of circulating naive or CXCR5+/− memory T cells. **Significant differences between CXCR5+ and CXCR5− memory cells. (B) IL-4/IFN-γ production capabilities of CXCR5+/− T cells during repeated T cell receptor activation (each cycle is composed of 4-d activation with anti-CD3 and anti-CD28 followed by 3-d resting in the presence of IL-2). (C) B cell help activity of circulating CXCR5+ T cells after T cell receptor activation. Naive (IgD+) or memory (IgD−) B cells (2 × 104) from peripheral blood were cultured for 14 d in the presence or absence of various numbers (103, 5 × 103, 104, 2.5 × 104, 5 × 104, and 105) of autologous T cells (CXCR5−CD45RA+, CXCR5+CD45RA−, or CXCR5− CD45RA−). Concentrations of IgG, IgA, and IgM in the culture supernatants were measured by ELISA. T cell numbers required for peak levels of antibody production varied among donors or experiments. One peak value with the best antibody production in each T cell group is shown. (D) Loss of CXCR5 expression during T cell receptor activation with anti-CD3 and anti-CD28. Error bars indicate SD of results from at least five different experiments (A). Representatives of three independent experiments are shown (B and D), and results from seven different donors (C) are shown.
Similar articles
- CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function.
Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. Schaerli P, et al. J Exp Med. 2000 Dec 4;192(11):1553-62. doi: 10.1084/jem.192.11.1553. J Exp Med. 2000. PMID: 11104798 Free PMC article. - Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression.
Rasheed AU, Rahn HP, Sallusto F, Lipp M, Müller G. Rasheed AU, et al. Eur J Immunol. 2006 Jul;36(7):1892-903. doi: 10.1002/eji.200636136. Eur J Immunol. 2006. PMID: 16791882 - Germinal centers regulate human Th2 development.
Johansson-Lindbom B, Ingvarsson S, Borrebaeck CA. Johansson-Lindbom B, et al. J Immunol. 2003 Aug 15;171(4):1657-66. doi: 10.4049/jimmunol.171.4.1657. J Immunol. 2003. PMID: 12902463 - CXCR5(+) T cells: follicular homing takes center stage in T-helper-cell responses.
Moser B, Schaerli P, Loetscher P. Moser B, et al. Trends Immunol. 2002 May;23(5):250-4. doi: 10.1016/s1471-4906(02)02218-4. Trends Immunol. 2002. PMID: 12102746 Review. No abstract available. - Lymphocyte traffic control by chemokines: follicular B helper T cells.
Moser B, Ebert L. Moser B, et al. Immunol Lett. 2003 Jan 22;85(2):105-12. doi: 10.1016/s0165-2478(02)00233-x. Immunol Lett. 2003. PMID: 12527215 Review.
Cited by
- T-follicular regulatory cells expand to control germinal center plasma cell output but fail to curb autoreactivity.
Fahlquist-Hagert C, Wittenborn TR, Pedersen MK, Jensen L, Degn SE. Fahlquist-Hagert C, et al. iScience. 2024 Sep 4;27(10):110887. doi: 10.1016/j.isci.2024.110887. eCollection 2024 Oct 18. iScience. 2024. PMID: 39319261 Free PMC article. - Generation of antigen-specific memory CD4 T cells by heterologous immunization enhances the magnitude of the germinal center response upon influenza infection.
Sircy LM, Ramstead AG, Gibbs LC, Joshi H, Baessler A, Mena I, García-Sastre A, Emerson LL, Fairfax KC, Williams MA, Hale JS. Sircy LM, et al. PLoS Pathog. 2024 Sep 16;20(9):e1011639. doi: 10.1371/journal.ppat.1011639. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39283916 Free PMC article. - Activation of circulating TFH17 cells associated with activated naive and double negative 2 B cell expansion, and disease activity in systemic lupus erythematosus patients.
Khunsri T, Thawornpan P, Tianpothong P, Suangtamai T, Ngamjanyaporn P, Leepiyasakulchai C, Wangriatisak K, Pisitkun P, Chootong P. Khunsri T, et al. Arthritis Res Ther. 2024 Sep 11;26(1):159. doi: 10.1186/s13075-024-03394-7. Arthritis Res Ther. 2024. PMID: 39261963 Free PMC article. - Role of TIM-3 in ovarian cancer: the forsaken cop or a new noble.
Chang X, Miao J. Chang X, et al. Front Immunol. 2024 Aug 13;15:1407403. doi: 10.3389/fimmu.2024.1407403. eCollection 2024. Front Immunol. 2024. PMID: 39206199 Free PMC article. Review. - Follicular Immune Landscaping Reveals a Distinct Profile of FOXP3hiCD4hi T Cells in Treated Compared to Untreated HIV.
Georgakis S, Orfanakis M, Brenna C, Burgermeister S, Del Rio Estrada PM, González-Navarro M, Torres-Ruiz F, Reyes-Terán G, Avila-Rios S, Luna-Villalobos YA, Chén OY, Pantaleo G, Koup RA, Petrovas C. Georgakis S, et al. Vaccines (Basel). 2024 Aug 12;12(8):912. doi: 10.3390/vaccines12080912. Vaccines (Basel). 2024. PMID: 39204036 Free PMC article.
References
- Metz D.P., Bottomly K. Function and regulation of memory CD4 T cells. Immunol. Res. 1999;19:127–141. - PubMed
- Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. - PubMed
- Carter L.L., Swain S.L. From naive to memory. Development and regulation of CD4+ T cell responses. Immunol. Res. 1998;18:1–13. - PubMed
- Butcher E.C., Williams M., Youngman K., Rott L., Briskin M. Lymphocyte trafficking and regional immunity. Adv. Immunol. 1999;72:209–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI37832/AI/NIAID NIH HHS/United States
- R37 GM037734/GM/NIGMS NIH HHS/United States
- R37 AI047822/AI/NIAID NIH HHS/United States
- GM37734/GM/NIGMS NIH HHS/United States
- AI47822/AI/NIAID NIH HHS/United States
- R01 GM037734/GM/NIGMS NIH HHS/United States
- R21 AI047822/AI/NIAID NIH HHS/United States
- R01 AI047822/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous