Functional dichotomy in natural killer cell signaling: Vav1-dependent and -independent mechanisms - PubMed (original) (raw)
Functional dichotomy in natural killer cell signaling: Vav1-dependent and -independent mechanisms
F Colucci et al. J Exp Med. 2001.
Abstract
The product of the protooncogene Vav1 participates in multiple signaling pathways and is a critical regulator of antigen-receptor signaling in B and T lymphocytes, but its role during in vivo natural killer (NK) cell differentiation is not known. Here we have studied NK cell development in Vav1-/- mice and found that, in contrast to T and NK-T cells, the absolute numbers of phenotypically mature NK cells were not reduced. Vav1-/- mice produced normal amounts of interferon (IFN)-gamma in response to Listeria monocytogenes and controlled early infection but showed reduced tumor clearance in vivo. In vitro stimulation of surface receptors in Vav1-/- NK cells resulted in normal IFN-gamma production but reduced tumor cell lysis. Vav1 was found to control activation of extracellular signal-regulated kinases and exocytosis of cytotoxic granules. In contrast, conjugate formation appeared to be only mildly affected, and calcium mobilization was normal in Vav1-/- NK cells. These results highlight fundamental differences between proximal signaling events in T and NK cells and suggest a functional dichotomy for Vav1 in NK cells: a role in cytotoxicity but not for IFN-gamma production.
Figures
Figure 1
Vav1 is dispensable for NK cell development. (A) Cells from spleen, marrow, and liver were stained with α-CD3FITC and α-NK1.1PE mAbs. Thymocytes were stained with α-CD3FITC, α-NK1.1PE, and α-HSABIO mAb, and a gate was set to exclude HSA+ immature cells. Figures in the dot plots indicate percentages of NK, NK-T, and T cells. Data are from one representative of eight independent experiments. (B) Spleen cells were stained with α-NK1.1PE, α-TCR-αβBIO, and α-CD19BIO and one of the indicated FITC-conjugated mAbs. Biotinylated Abs were revealed with streptavidin–TRI, and only NK1.1+ TCR-αβ−CD19− NK cells were included in the analysis. Figures show percentage of NK cells staining positive for FITC. No significant differences were detected between wt and Vav1 − _/_− NK cells. Results are from one representative of five independent experiments.
Figure 1
Vav1 is dispensable for NK cell development. (A) Cells from spleen, marrow, and liver were stained with α-CD3FITC and α-NK1.1PE mAbs. Thymocytes were stained with α-CD3FITC, α-NK1.1PE, and α-HSABIO mAb, and a gate was set to exclude HSA+ immature cells. Figures in the dot plots indicate percentages of NK, NK-T, and T cells. Data are from one representative of eight independent experiments. (B) Spleen cells were stained with α-NK1.1PE, α-TCR-αβBIO, and α-CD19BIO and one of the indicated FITC-conjugated mAbs. Biotinylated Abs were revealed with streptavidin–TRI, and only NK1.1+ TCR-αβ−CD19− NK cells were included in the analysis. Figures show percentage of NK cells staining positive for FITC. No significant differences were detected between wt and Vav1 − _/_− NK cells. Results are from one representative of five independent experiments.
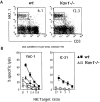
Figure 2
Vav1 is required for antitumor activity but not for antibacterial activity. (A) Mice (five wt, four Vav1 − _/_−, three _Rag2/_γc_−/_−) were injected intravenously with 105 51Cr-labeled RMA-S cells. 4 h after injection, the residual radioactivity was measured in the lungs and is expressed as percentage of the total radioactivity. Data are representative of three separate experiments. (B) Mice (eight wt, five Vav1 − _/_−, six _Rag2/_γc_−/_−) were injected intravenously with 2.5 × 105 labeled RMA-S cells. 48 h after injection, cells in the peritoneal exudate were collected, enumerated, and stained with α-CD3 and α-H-2Kk. Residual RMA-S cells were discriminated by virtue of size and surface phenotype (large CD3+H-2K− cells). (C) Mice (six wt, six Vav1 − _/_−, four _Rag2/_γc_−/_−) were injected intravenously with 104 L.m., and 48 h later liver CFUs were enumerated. (D) The sera from mice infected with L.m. (10 wt, 11 Vav1 − _/_−, 4 _Rag2/_γc_−/_−) were collected after mice were killed, and IFN-γ was detected by standard ELISA technique. P values (Student's t test) are expressed for Vav1 − _/_− versus wt. White bars, _Rag2/_γc_−/_−; black bars, wt; gray bars, Vav1 − _/_−.
Figure 3
Vav1 regulates NK cell cytotoxicity. (A) Splenocytes were enriched for NK cells by passage on nylon wool columns, and percentages of NK cells are shown for a representative experiment. (B) NK-enriched splenocytes from poly(I:C)-injected mice (squares) or from untreated mice (triangles) were used to test natural cytotoxicity versus YAC-1 and IC-21 tumor targets at the indicated NK/target ratios. (C) Splenocytes were enriched for NK cells and expanded in IL-2. The percentages of NK cells are shown for a representative experiment. (D) IL-2–activated NK cells were tested for cytolytic activity versus YAC-1, Con A–activated B6.β2m − _/_− blasts, Tap − _/_− RMA-S cells (both MHC class I deficient); control experiments included Con A–activated wt blasts and RMA cells (both expressing normal levels of MHC class I). (E) ADCC of Ab-coated EL4 cells and for IL-2–activated NK cells were tested for reverse ADCC of FcR+ P815 cells in presence of the indicated Abs. Control experiments included non–Ab-coated EL4 and P815 cells. Each experiment is representative of three.
Figure 3
Vav1 regulates NK cell cytotoxicity. (A) Splenocytes were enriched for NK cells by passage on nylon wool columns, and percentages of NK cells are shown for a representative experiment. (B) NK-enriched splenocytes from poly(I:C)-injected mice (squares) or from untreated mice (triangles) were used to test natural cytotoxicity versus YAC-1 and IC-21 tumor targets at the indicated NK/target ratios. (C) Splenocytes were enriched for NK cells and expanded in IL-2. The percentages of NK cells are shown for a representative experiment. (D) IL-2–activated NK cells were tested for cytolytic activity versus YAC-1, Con A–activated B6.β2m − _/_− blasts, Tap − _/_− RMA-S cells (both MHC class I deficient); control experiments included Con A–activated wt blasts and RMA cells (both expressing normal levels of MHC class I). (E) ADCC of Ab-coated EL4 cells and for IL-2–activated NK cells were tested for reverse ADCC of FcR+ P815 cells in presence of the indicated Abs. Control experiments included non–Ab-coated EL4 and P815 cells. Each experiment is representative of three.
Figure 3
Vav1 regulates NK cell cytotoxicity. (A) Splenocytes were enriched for NK cells by passage on nylon wool columns, and percentages of NK cells are shown for a representative experiment. (B) NK-enriched splenocytes from poly(I:C)-injected mice (squares) or from untreated mice (triangles) were used to test natural cytotoxicity versus YAC-1 and IC-21 tumor targets at the indicated NK/target ratios. (C) Splenocytes were enriched for NK cells and expanded in IL-2. The percentages of NK cells are shown for a representative experiment. (D) IL-2–activated NK cells were tested for cytolytic activity versus YAC-1, Con A–activated B6.β2m − _/_− blasts, Tap − _/_− RMA-S cells (both MHC class I deficient); control experiments included Con A–activated wt blasts and RMA cells (both expressing normal levels of MHC class I). (E) ADCC of Ab-coated EL4 cells and for IL-2–activated NK cells were tested for reverse ADCC of FcR+ P815 cells in presence of the indicated Abs. Control experiments included non–Ab-coated EL4 and P815 cells. Each experiment is representative of three.
Figure 3
Vav1 regulates NK cell cytotoxicity. (A) Splenocytes were enriched for NK cells by passage on nylon wool columns, and percentages of NK cells are shown for a representative experiment. (B) NK-enriched splenocytes from poly(I:C)-injected mice (squares) or from untreated mice (triangles) were used to test natural cytotoxicity versus YAC-1 and IC-21 tumor targets at the indicated NK/target ratios. (C) Splenocytes were enriched for NK cells and expanded in IL-2. The percentages of NK cells are shown for a representative experiment. (D) IL-2–activated NK cells were tested for cytolytic activity versus YAC-1, Con A–activated B6.β2m − _/_− blasts, Tap − _/_− RMA-S cells (both MHC class I deficient); control experiments included Con A–activated wt blasts and RMA cells (both expressing normal levels of MHC class I). (E) ADCC of Ab-coated EL4 cells and for IL-2–activated NK cells were tested for reverse ADCC of FcR+ P815 cells in presence of the indicated Abs. Control experiments included non–Ab-coated EL4 and P815 cells. Each experiment is representative of three.
Figure 4
Vav1 is dispensable for NK cell–mediated IFN-γ production. (A) Sorted IL-2–activated NK cells were incubated with different plate-bound antibodies or with IL-12. After 24 h, the amount of IFN-γ produced was measured by a standard ELISA. One representative experiment of two independent experiments performed. (B) Sorted IL-2–activated NK cells were incubated at 37°C with the indicated stimuli and thereafter stained for intracellular IFN-γ content.
Figure 4
Vav1 is dispensable for NK cell–mediated IFN-γ production. (A) Sorted IL-2–activated NK cells were incubated with different plate-bound antibodies or with IL-12. After 24 h, the amount of IFN-γ produced was measured by a standard ELISA. One representative experiment of two independent experiments performed. (B) Sorted IL-2–activated NK cells were incubated at 37°C with the indicated stimuli and thereafter stained for intracellular IFN-γ content.
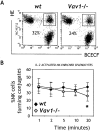
Figure 5
Vav1 plays a minor role in NK cell conjugate formation. (A) BCECF-labeled NK cells were allowed to form conjugates with HE-labeled YAC-1 targets for 20 min at 37°C and immediately analyzed by flow cytometry. Double-positive cells are conjugates, and the percentages (NK cells bound to targets out of the total NK cells) for the two genotypes are indicated for one representative of six independent experiments. Nonspecific aggregates were reproducibly <1%. (B) Kinetics of conjugate formation. Values at each time point were analyzed statistically, and significant differences (P < 0.05 by Student's t test) are indicated by an asterisk.
Figure 6
T cells but not NK cells require Vav1 to initiate calcium flux. Purified NK cells and T cells were stimulated with the primary mAbs (anti-NK1.1, anti-CD16, or anti-CD3), and analysis was done for 45 s to set the baseline levels of intracellular calcium. Acquisition was interrupted once to add cross-linking goat F(ab′)2 Abs and again to add 1 μg/ml of ionomycin. Cells were analyzed by LSR-FACS®. Intracellular calcium is proportional to the FL5/FL4 ratio.
Figure 7
Vav1 controls ERK activation and granule exocytosis. (A) Purified NK and T cells were incubated with the indicated mAb and stimulated with cross-linking Abs. Cell lysates were immunoblotted with an mAb specific for the phosphorylated forms of ERK1/2. Total amounts of inactive ERK1/2 were assessed by polyclonal anti-ERK1/2. Stimulation with PMA generated comparable levels of phosphorylated ERK1/2 in wt and Vav1 − _/_− cells. (B) Equal numbers of NK cells were stimulated with YAC-1 targets for 2 h and 30 min at 37°C. Supernatants were collected, and the specific release of esterase (granzyme A) was measured by using a BLT colorimetric assay, where the maximum release of esterase was obtained by freezing and thawing NK cells. Mean values ± SDs are shown from five independent experiments.
Figure 7
Vav1 controls ERK activation and granule exocytosis. (A) Purified NK and T cells were incubated with the indicated mAb and stimulated with cross-linking Abs. Cell lysates were immunoblotted with an mAb specific for the phosphorylated forms of ERK1/2. Total amounts of inactive ERK1/2 were assessed by polyclonal anti-ERK1/2. Stimulation with PMA generated comparable levels of phosphorylated ERK1/2 in wt and Vav1 − _/_− cells. (B) Equal numbers of NK cells were stimulated with YAC-1 targets for 2 h and 30 min at 37°C. Supernatants were collected, and the specific release of esterase (granzyme A) was measured by using a BLT colorimetric assay, where the maximum release of esterase was obtained by freezing and thawing NK cells. Mean values ± SDs are shown from five independent experiments.
Similar articles
- Differential requirements for Vav proteins in DAP10- and ITAM-mediated NK cell cytotoxicity.
Cella M, Fujikawa K, Tassi I, Kim S, Latinis K, Nishi S, Yokoyama W, Colonna M, Swat W. Cella M, et al. J Exp Med. 2004 Sep 20;200(6):817-23. doi: 10.1084/jem.20031847. Epub 2004 Sep 13. J Exp Med. 2004. PMID: 15365099 Free PMC article. - Dependence of both spontaneous and antibody-dependent, granule exocytosis-mediated NK cell cytotoxicity on extracellular signal-regulated kinases.
Trotta R, Puorro KA, Paroli M, Azzoni L, Abebe B, Eisenlohr LC, Perussia B. Trotta R, et al. J Immunol. 1998 Dec 15;161(12):6648-56. J Immunol. 1998. PMID: 9862693 - Vav-1 regulates NK T cell development and NK cell cytotoxicity.
Chan G, Hanke T, Fischer KD. Chan G, et al. Eur J Immunol. 2001 Aug;31(8):2403-10. doi: 10.1002/1521-4141(200108)31:8<2403::aid-immu2403>3.0.co;2-o. Eur J Immunol. 2001. PMID: 11500824 - Vav1: a key signal transducer downstream of the TCR.
Tybulewicz VL, Ardouin L, Prisco A, Reynolds LF. Tybulewicz VL, et al. Immunol Rev. 2003 Apr;192:42-52. doi: 10.1034/j.1600-065x.2003.00032.x. Immunol Rev. 2003. PMID: 12670394 Review. - Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis.
Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Moretta A, et al. Annu Rev Immunol. 2001;19:197-223. doi: 10.1146/annurev.immunol.19.1.197. Annu Rev Immunol. 2001. PMID: 11244035 Review.
Cited by
- Controlling natural killer cell responses: integration of signals for activation and inhibition.
Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Long EO, et al. Annu Rev Immunol. 2013;31:227-58. doi: 10.1146/annurev-immunol-020711-075005. Annu Rev Immunol. 2013. PMID: 23516982 Free PMC article. Review. - NK cell-activating receptors require PKC-theta for sustained signaling, transcriptional activation, and IFN-gamma secretion.
Tassi I, Cella M, Presti R, Colucci A, Gilfillan S, Littman DR, Colonna M. Tassi I, et al. Blood. 2008 Nov 15;112(10):4109-16. doi: 10.1182/blood-2008-02-139527. Epub 2008 Sep 10. Blood. 2008. PMID: 18784374 Free PMC article. - NKG2A genetic deletion promotes human primary NK cell anti-tumor responses better than an anti-NKG2A monoclonal antibody.
Gong Y, Germeraad WTV, Zhang X, Wu N, Li B, Janssen L, He Z, Gijbels MJJ, Wu B, Gijsbers BLMG, Olieslagers TI, Bos GMJ, Zheng L, Klein Wolterink RGJ. Gong Y, et al. Mol Ther. 2024 Aug 7;32(8):2711-2727. doi: 10.1016/j.ymthe.2024.06.034. Epub 2024 Jun 27. Mol Ther. 2024. PMID: 38943249 Free PMC article. - Longitudinal multiparameter assay of lymphocyte interactions from onset by microfluidic cell pairing and culture.
Dura B, Servos MM, Barry RM, Ploegh HL, Dougan SK, Voldman J. Dura B, et al. Proc Natl Acad Sci U S A. 2016 Jun 28;113(26):E3599-608. doi: 10.1073/pnas.1515364113. Epub 2016 Jun 14. Proc Natl Acad Sci U S A. 2016. PMID: 27303033 Free PMC article. - β2 integrin induces TCRζ-Syk-phospholipase C-γ phosphorylation and paxillin-dependent granule polarization in human NK cells.
March ME, Long EO. March ME, et al. J Immunol. 2011 Mar 1;186(5):2998-3005. doi: 10.4049/jimmunol.1002438. Epub 2011 Jan 26. J Immunol. 2011. PMID: 21270398 Free PMC article.
References
- Collins T.L., Deckert M., Altman A. Views on Vav. Immunol. Today. 1997;18:221–225. - PubMed
- Bustelo X.R., Ledbetter J.A., Barbacid M. Product of vav proto-oncogene defines a new class of tyrosine protein kinase substrates. Nature. 1992;356:68–71. - PubMed
- Margolis B., Hu P., Katzav S., Li W., Olive R.J.M., Ullrich A., Weiss A., Schlessinger J. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 1992;356:71–74. - PubMed
- Tarakhovsky A., Turner M., Schaal S., Mee P.J., Duddy L.P., Rajewsky K., Tybulewicz V.L. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature. 1995;374:467–470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous