The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex - PubMed (original) (raw)
The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex
J P Johansen et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Numerous human and animal studies indirectly implicate neurons in the anterior cingulate cortex (ACC) in the encoding of the affective consequences of nociceptor stimulation. No causal evidence, however, has been put forth linking the ACC specifically to this function. Using a rodent pain assay that combines the hind-paw formalin model with the place-conditioning paradigm, we measured a learned behavior that directly reflects the affective component of pain in the rat (formalin-induced conditioned place avoidance) concomitantly with "acute" formalin-induced nociceptive behaviors (paw lifting, licking, and flinching) that reflect the intensity and localization of the nociceptive stimulus. Destruction of neurons originating from the rostral, but not caudal, ACC reduced formalin-induced conditioned place avoidance without reducing acute pain-related behaviors. These results provide evidence indicating that neurons in the ACC are necessary for the "aversiveness" of nociceptor stimulation.
Figures
Figure 1
Photomicrographs of representative coronal sections through the rostral (A and B) and caudal (C and D) ACC. Sections were stained with cresyl violet. (A) Section from a rostral ACC sham lesion animal as compared with a section taken from a rostral ACC lesion animal (B) at the same antero-posterior level. Sections taken from the same antero-posterior level of caudal ACC sham lesion and caudal ACC lesion animals are compared in C and D, respectively. Clear lesion areas are evidenced by neuronal cell loss and by the proliferation of smaller glial cells (especially apparent around the borders of the lesions).
Figure 2
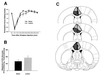
Formalin-induced pain behaviors and brain lesion maps associated with rats with rostral ACC lesions (n = 8) and rostral ACC sham lesions (n = 10). Data are represented as mean ± SEM. Acute formalin-induced nociceptive scores (rating scale) are shown in A. Magnitude of CPA scores are shown in B. Representations of the largest and smallest lesions in the rostral ACC lesion group are shown in C. Mean percent damage calculations for each hemisphere and a bilateral mean for each experimental group are as follows: left hemisphere, 71% ± 4%; right hemisphere, 51% ± 6%; mean, 62% ± 4%. Rostral ACC lesions completely abolished F-CPA (B) without causing a concomitant reduction in acute formalin-induced nociceptive behaviors (A). (A) *, P < 0.05, Tukey's honestly significant difference test, as compared with rats with sham lesions of the rostral ACC. (B) *, P < 0.05, Student's t test, as compared with rats with sham lesions of the rostral ACC.
Figure 3
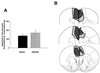
Formalin-induced pain behaviors and brain lesion maps associated with rats with caudal ACC lesions (n = 8) and caudal ACC sham lesions (n = 11). Data are represented as mean ± SEM. Acute formalin-induced nociceptive scores are shown in A. Magnitude of CPA scores are shown in B. Representations of the largest and smallest lesions in the caudal ACC lesion group are shown in C. Mean “percent damage” calculations for each hemisphere and a bilateral mean for each experimental group are as follows: left hemisphere, 82% ± 5%; right hemisphere, 67% ± 5%; mean, 74% ± 4%. Caudal ACC lesions had no effect on either acute formalin-induced nociceptive behaviors (A) or F-CPA (B).
Figure 4
CPA produced by a non-nociceptive stimulus (the κ-opioid receptor agonist U69,593) in rats with rostral ACC lesions (n = 9) and rostral ACC sham lesions (n = 8). Data are represented as mean ± SEM. U69,593-induced magnitude of CPA scores are shown in A. Representations of the largest and smallest lesions in the rostral ACC lesion group are shown in B. Mean percent damage calculations for each hemisphere and a bilateral mean for each experimental group are as follows: left hemisphere, 84% ± 4%; right hemisphere, 60% ± 3%; mean, 69% ± 3%. Rostral ACC lesions had no effect on U69,593-induced CPA.
Similar articles
- NMDA NR2A and NR2B receptors in the rostral anterior cingulate cortex contribute to pain-related aversion in male rats.
Li TT, Ren WH, Xiao X, Nan J, Cheng LZ, Zhang XH, Zhao ZQ, Zhang YQ. Li TT, et al. Pain. 2009 Nov;146(1-2):183-93. doi: 10.1016/j.pain.2009.07.027. Epub 2009 Aug 19. Pain. 2009. PMID: 19695778 Free PMC article. - Contributions of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats.
Gao YJ, Ren WH, Zhang YQ, Zhao ZQ. Gao YJ, et al. Pain. 2004 Jul;110(1-2):343-53. doi: 10.1016/j.pain.2004.04.030. Pain. 2004. PMID: 15275785 - Glutamatergic activation of anterior cingulate cortex mediates the affective component of visceral pain memory in rats.
Yan N, Cao B, Xu J, Hao C, Zhang X, Li Y. Yan N, et al. Neurobiol Learn Mem. 2012 Jan;97(1):156-64. doi: 10.1016/j.nlm.2011.11.003. Epub 2011 Nov 15. Neurobiol Learn Mem. 2012. PMID: 22107830 - A new perspective on the anterior cingulate cortex and affective pain.
Xiao X, Zhang YQ. Xiao X, et al. Neurosci Biobehav Rev. 2018 Jul;90:200-211. doi: 10.1016/j.neubiorev.2018.03.022. Epub 2018 Apr 24. Neurosci Biobehav Rev. 2018. PMID: 29698697 Review. - Contributions of anterior cingulate cortex to behaviour.
Devinsky O, Morrell MJ, Vogt BA. Devinsky O, et al. Brain. 1995 Feb;118 ( Pt 1):279-306. doi: 10.1093/brain/118.1.279. Brain. 1995. PMID: 7895011 Review.
Cited by
- Electrophysiology as a Tool to Decipher the Network Mechanism of Visceral Pain in Functional Gastrointestinal Disorders.
Alam MJ, Chen JDZ. Alam MJ, et al. Diagnostics (Basel). 2023 Feb 8;13(4):627. doi: 10.3390/diagnostics13040627. Diagnostics (Basel). 2023. PMID: 36832115 Free PMC article. Review. - Parallel Spinal Pathways for Transmitting Reflexive and Affective Dimensions of Nocifensive Behaviors Evoked by Selective Activation of the Mas-Related G Protein-Coupled Receptor D-Positive and Transient Receptor Potential Vanilloid 1-Positive Subsets of Nociceptors.
Wang LB, Su XJ, Wu QF, Xu X, Wang XY, Chen M, Ye JR, Maimaitiabula A, Liu XQ, Sun W, Zhang Y. Wang LB, et al. Front Cell Neurosci. 2022 May 24;16:910670. doi: 10.3389/fncel.2022.910670. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35693883 Free PMC article. - Contribution of anterior cingulate cortex and descending pain inhibitory system to analgesic effect of lemon odor in mice.
Ikeda H, Takasu S, Murase K. Ikeda H, et al. Mol Pain. 2014 Feb 20;10:14. doi: 10.1186/1744-8069-10-14. Mol Pain. 2014. PMID: 24555533 Free PMC article. - Neuroinflammation in the anterior cingulate cortex: the potential supraspinal mechanism underlying the mirror-image pain following motor fiber injury.
Li QY, Chen SX, Liu JY, Yao PW, Duan YW, Li YY, Zang Y. Li QY, et al. J Neuroinflammation. 2022 Jun 20;19(1):162. doi: 10.1186/s12974-022-02525-8. J Neuroinflammation. 2022. PMID: 35725625 Free PMC article.
References
- Fields H L. Pain. 1999;6,Suppl.:S61–S69. - PubMed
- Melzack R, Casey K L. In: The Skin Senses. Kenshalo D, editor. Springfield, IL: Thomas; 1968. pp. 423–439.
- Melzack R. Pain. 1975;1:277–299. - PubMed
- Price D D. Science. 2000;288:1769–1772. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical