Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response - PubMed (original) (raw)
Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response
J Kipnis et al. J Neurosci. 2001.
Abstract
Injury to the CNS is often followed by a spread of damage (secondary degeneration), resulting in neuronal losses that are substantially greater than might have been predicted from the severity of the primary insult. Studies in our laboratory have shown that injured CNS neurons can benefit from active or passive immunization with CNS myelin-associated antigens. The fact that autoimmune T-cells can be both beneficial and destructive, taken together with the established phenomenon of genetic predisposition to autoimmune diseases, raises the question: will genetic predisposition to autoimmune diseases affect the outcome of traumatic insult to the CNS? Here we show that the survival rate of retinal ganglion cells in adult mice or rats after crush injury of the optic nerve or intravitreal injection of a toxic dosage of glutamate is up to twofold higher in strains that are resistant to the CNS autoimmune disease experimental autoimmune encephalomyelitis (EAE) than in susceptible strains. The difference was found to be attributed, at least in part, to a beneficial T-cell response that was spontaneously evoked after CNS insult in the resistant but not in the susceptible strains. In animals of EAE-resistant but not of EAE-susceptible strains devoid of mature T-cells (as a result of having undergone thymectomy at birth), the numbers of surviving neurons after optic nerve injury were significantly lower (by 60%) than in the corresponding normal animals. Moreover, the rate of retinal ganglion cell survival was higher when the optic nerve injury was preceded by an unrelated CNS (spinal cord) injury in the resistant strains but not in the susceptible strains. It thus seems that, in normal animals of EAE-resistant strains (but not of susceptible strains), the injury evokes an endogenous protective response that is T-cell dependent. These findings imply that a protective T-cell-dependent response and resistance to autoimmune disease are regulated by a common mechanism. The results of this study compel us to modify our understanding of autoimmunity and autoimmune diseases, as well as the role of autoimmunity in non-autoimmune CNS disorders. They also obviously have far-reaching clinical implications in terms of prognosis and individual therapy.
Figures
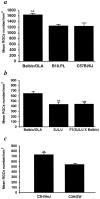
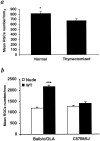
Fig. 1.
Survival rate of retinal ganglion cells after optic nerve injury is associated with resistance to the autoimmune disease EAE. The RGCs of adult C57BL/6J, B10.PL, BALB/c/OLA (a), BALB/c/OLA, SJL/J, F1(SJL/J×BALB/c/OLA) (b), and C3H.SW and C3H/HeJ (c) mice were retrogradely labeled with the neurotracer dye FluoroGold injected stereotactically into the superior colliculus. Three days later, the mice were subjected to severe crush injury of the intraorbital portion of the optic nerve. One (a) or 2 (b, c) weeks after optic nerve crush injury, the retina was detached from the eye, prepared as a flattened whole mount, and examined for labeled RGCs by fluorescence microscopy. One week after the injury, survival rates were significantly lower in B10.PL and C57BL/6J mice than in resistant BALB/c/OLA mice (p < 0.01) (a). Similar results were obtained in SJL/J (p < 0.01) and F1(SJL/J×BALB/c/OLA) mice (p < 0.001), in which RGC survival rates were significantly lower than in the resistant BALB/c/OLA mice (b). RGC survival rates were significantly higher in C3H/HeJ mice (EAE-resistant) than in congenic (EAE-susceptible) C3H.SW mice (p < 0.001) (c). The average numbers of RGCs on the uninjured side were similar (∼3500 RGCs per square millimeter) in all mouse strains. **p < 0.01; ***p < 0.001; Student's_t_ test.)
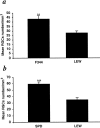
Fig. 2.
Survival of neurons after partial crush injury of the optic nerve in Lew, SPD, and F344 rats. The optic nerves of adult Lew, SPD, and F344 rats were subjected to a partial crush injury 1–2 mm from the eye, using calibrated cross-action forceps. Two weeks later, the optic nerves were exposed for the second time, and the fluorescent dye 4-Di-10-Asp was applied distally to the injury site. Five days after dye application, the retinas were detached from the eyes and prepared for fluorescence microscopy as described in Figure 1. The amount of endogenous neuroprotection was significantly greater in F344 and SPD rats (p < 0.01 and_p_ < 0.001, respectively), known to be resistant to EAE induction. **p < 0.01; ***p < 0.001; Student's t test.
Fig. 3.
T-cell dependence of physiological differences in the response to optic nerve crush injury in EAE-resistant and EAE-susceptible rats. a, Adult Lew and SPD rats, which had undergone thymectomy 1 d after birth, and normal adult control rats were subjected to optic nerve crush, and their RGCs were counted 2 weeks later, as described in Figure 2. Significantly fewer labeled RGCs were seen in the thymectomized SPD rats than in the normal SPD rats (p < 0.01). In Lew rats, the opposite was seen, but the effect was not significant (_p_> 0.1). b, Lew and SPD rats, thymectomized 1 d after birth, were subjected to contusive injury of the spinal cord at the level of T7 or T9 using the NYU impactor. Sham-operated rats were laminectomized but not contused. After 2 weeks, both sham-operated and contused rats were subjected to optic nerve crush and counting of surviving RGCs, as described in Figure 2. Significantly more labeled RGCs were seen in the preinjured SPD rats than in the sham-operated SPD controls (p < 0.05). In Lew rats, differences in the number of labeled RGCs between the preinjured and the sham-operated rats after optic nerve injury were not significant (_p_ > 0.1). *p < 0.05; **p < 0.01; Student's t test.
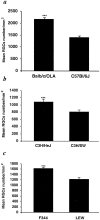
Fig. 4.
Glutamate toxicity is correlated with susceptibility to autoimmune disease. Glutamate (200 nmol) was injected intravitreally into EAE-susceptible and EAE-resistant strains of mice (C57BL/6J and BALB/c/OLA, respectively), the congenic mice C3H.SW and C3H/HeJ (differing in H-2 haplotype), and rats (Lew and F344). One week after glutamate injection, more surviving RGCs were found in mice of EAE-resistant strains (C3H/HeJ and BALB/c/OLA) than in susceptible strains (C3H.SW and C57BL/6J) (p < 0.001 in both a and b). As with the mice, significantly more RGCs were seen in the retinas of EAE-resistant rats (F344) than in the retinas of EAE-susceptible rats (Lew) (c, p < 0.001). No differences in the numbers of RGCs between susceptible and resistant strains were observed on the uninjured side in either rats or mice. ***p< 0.001; Student's t test.
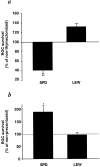
Fig. 5.
Glutamate evokes a self-protective autoimmune response in mice and rats genetically resistant to EAE induction. Normal adult SPD rats and adult SPD rats that had undergone thymectomy at birth were injected intravitreally with glutamate (200 nmol). One week later, the numbers of surviving RGCs were determined by retrograde labeling with 4-Di-10-Asp. Significantly fewer surviving RGCs were seen in the thymectomized rats than in the normal controls (a, p < 0.01). After glutamate toxicity, nude mice of a genetically susceptible strain (C57BL/6J) showed no differences in RGC survival rates compared with their wild-type counterparts, whereas RGC survival rates in nude mice of a genetically resistant strain were significantly decreased (p < 0.001) (b). *p < 0.05; ***p < 0.001; Student's_t_ test.
Similar articles
- Protective autoimmunity is a physiological response to CNS trauma.
Yoles E, Hauben E, Palgi O, Agranov E, Gothilf A, Cohen A, Kuchroo V, Cohen IR, Weiner H, Schwartz M. Yoles E, et al. J Neurosci. 2001 Jun 1;21(11):3740-8. doi: 10.1523/JNEUROSCI.21-11-03740.2001. J Neurosci. 2001. PMID: 11356861 Free PMC article. - Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system.
Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Kipnis J, et al. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15620-5. doi: 10.1073/pnas.232565399. Epub 2002 Nov 12. Proc Natl Acad Sci U S A. 2002. PMID: 12429857 Free PMC article. - Resistance of retinal ganglion cells to an increase in intraocular pressure is immune-dependent.
Bakalash S, Kipnis J, Yoles E, Schwartz M. Bakalash S, et al. Invest Ophthalmol Vis Sci. 2002 Aug;43(8):2648-53. Invest Ophthalmol Vis Sci. 2002. PMID: 12147598 - Autoimmunity can benefit self-maintenance.
Schwartz M, Cohen IR. Schwartz M, et al. Immunol Today. 2000 Jun;21(6):265-8. doi: 10.1016/s0167-5699(00)01633-9. Immunol Today. 2000. PMID: 10825737 Review. - Autoimmune maintenance and neuroprotection of the central nervous system.
Cohen IR, Schwartz M. Cohen IR, et al. J Neuroimmunol. 1999 Dec;100(1-2):111-4. doi: 10.1016/s0165-5728(99)00190-3. J Neuroimmunol. 1999. PMID: 10695721 Review.
Cited by
- Traumatic optic neuropathy: a review.
Kumaran AM, Sundar G, Chye LT. Kumaran AM, et al. Craniomaxillofac Trauma Reconstr. 2015 Mar;8(1):31-41. doi: 10.1055/s-0034-1393734. Epub 2014 Nov 25. Craniomaxillofac Trauma Reconstr. 2015. PMID: 25709751 Free PMC article. Review. - Debate: "is increasing neuroinflammation beneficial for neural repair?".
Crutcher KA, Gendelman HE, Kipnis J, Perez-Polo JR, Perry VH, Popovich PG, Weaver LC. Crutcher KA, et al. J Neuroimmune Pharmacol. 2006 Sep;1(3):195-211. doi: 10.1007/s11481-006-9021-7. Epub 2006 May 23. J Neuroimmune Pharmacol. 2006. PMID: 18040798 Review. No abstract available. - Vaccination with a Nogo-A-derived peptide after incomplete spinal-cord injury promotes recovery via a T-cell-mediated neuroprotective response: comparison with other myelin antigens.
Hauben E, Ibarra A, Mizrahi T, Barouch R, Agranov E, Schwartz M. Hauben E, et al. Proc Natl Acad Sci U S A. 2001 Dec 18;98(26):15173-8. doi: 10.1073/pnas.011585298. Proc Natl Acad Sci U S A. 2001. PMID: 11752461 Free PMC article. - Endorphinergic Enhancement Attenuation of Post-traumatic Stress Disorder (PTSD) via Activation of Neuro-immunological Function in the Face of a Viral Pandemic.
Blum K, Modestino EJ, Baron D, Brewer R, Thanos P, Elman I, Badgaiyan RD, Downs BW, Bagchi D, McLaughlin T, Bowirrat A, Roy AK, Gold MS. Blum K, et al. Curr Psychopharmacol. 2021 Aug 1;10(2):86-97. doi: 10.2174/2211556009999210104221215. Curr Psychopharmacol. 2021. PMID: 34466374 Free PMC article. - How and why do T cells and their derived cytokines affect the injured and healthy brain?
Filiano AJ, Gadani SP, Kipnis J. Filiano AJ, et al. Nat Rev Neurosci. 2017 Jun;18(6):375-384. doi: 10.1038/nrn.2017.39. Epub 2017 Apr 27. Nat Rev Neurosci. 2017. PMID: 28446786 Free PMC article. Review.
References
- Arnon R. Experimental allergic encephalomyelitis—susceptibility and suppression. Immunol Rev. 1981;55:5–30. - PubMed
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996a;139:244–256. - PubMed
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996b;13:343–359. - PubMed
- Bebo BF, Zelinka-Vincent E, Adamus G, Amundson D, Vandenbark AA, Offner H. Gonadal hormones influence the immune response to PLP 139–151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 1998;84:122–130. - PubMed
- Bellone M, Ostlie N, Lei S, Conti-Tronconi BM. Experimental myasthenia gravis in congenic mice. Sequence mapping and H-2 restriction of T helper epitopes on the alpha subunits of Torpedo californica and murine acetylcholine receptors. Eur J Immunol. 1991;21:2303–2310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases