MIP-1alpha, MCP-1, GM-CSF, and TNF-alpha control the immune cell response that mediates rapid phagocytosis of myelin from the adult mouse spinal cord - PubMed (original) (raw)
MIP-1alpha, MCP-1, GM-CSF, and TNF-alpha control the immune cell response that mediates rapid phagocytosis of myelin from the adult mouse spinal cord
S S Ousman et al. J Neurosci. 2001.
Abstract
The slow immune response in the adult mammalian CNS results in slow myelin phagocytosis along degenerating white matter after injury. This has important consequences for axon regeneration because of the presence of axon growth inhibitors in myelin. In addition, abnormal immune cell responses in the CNS lead to demyelinating disease. Lysophosphatidylcholine (LPC) can induce an inflammatory response in the CNS, producing rapid demyelination without much damage to adjacent cells. In this study, we searched for the molecular switches that turn on this immune cell response. Using reverse transcription PCR analysis, we show that mRNA expression of macrophage inflammatory protein-1alpha (MIP-1alpha), macrophage chemotactic protein-1 (MCP-1), granulocyte macrophage-colony stimulating factor (GM-CSF), and tumor necrosis factor-alpha (TNF-alpha) in the spinal cord is rapidly and transiently upregulated after intraspinal injection of LPC. Neutralizing these signaling molecules with function-blocking antibodies suppresses recruitment of T-cells, neutrophils, and monocytes into the spinal cord, as well as significantly reduces the number of phagocytic macrophages and the demyelination induced by LPC. These findings will have important implications for CNS regeneration and demyelinating disease.
Figures
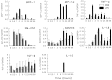
Fig. 1.
Time course of changes in cytokine and chemokine mRNA levels in the adult mouse spinal cord after injection of LPC and PBS. MCP-1, MIP-1α, TNF-α, and GM-CSF mRNA levels increased above that in PBS controls within 0.5–3 hr after LPC (▪) injection compared with animals injected with PBS (■). Levels reached a peak between 0.5 and 6 hr. Upregulation of RANTES, IL-1β, and TGF-β mRNA was seen later between 12 and 96 hr. An upregulation of IL-10 was seen only in the LPC group at one time point, 12 hr. IL-1α, IFN-γ, and IL-4 mRNA was not detected at any time point in either the LPC- or PBS-injected mice. Graphs represent densitometric values that were normalized to GAPDH. N indicates normal uninjured spinal cord. Mean ± SEM.
Fig. 2.
Neutralizing MIP-1α, MCP-1, GM-CSF, and TNF-α individually with function-blocking antibodies reduces the number of LPC-induced phagocytic macrophages. Graphs show the number of Mac-1+ phagocytic macrophages in the white matter 4 d after injection of LPC along with blocking antibodies (▪) or LPC plus control antibodies (■). Blocking each of these molecules resulted in a statistically significant reduction in the number of phagocytic macrophages compared with controls. Anti-MIP-1α injection produced the greatest reduction, ∼10-fold. Mean ± SEM (*p < 0.003, MCP-1; p < 0.003, MIP-1α; p < 0.02, TNF-α;p < 0.01, GM-CSF); n = 4 animals.
Fig. 3.
Neutralizing MIP-1α, MCP-1, GM-CSF, and TNF-α together results in marked reduction in the number of LPC-induced phagocytic macrophages. A, B, Mac-1 immunohistochemistry of longitudinal sections of the adult mouse spinal cord 4 d after injection of LPC along with either control antibodies (A) or all four neutralizing antibodies (B). In control animals, large, round Mac-1+ macrophages that have clear areas in the cytoplasm are seen (A). In contrast, very few of these Mac-1+ cells are seen in animals injected with LPC plus blocking antibodies (B). Sections were counterstained with Neutral Red. Scale bar, 80 μm. C, Quantification of Mac-1+ macrophages in the spinal cord 4 d after injections of LPC along with either control antibodies (■) or blocking antibodies (▪). Blocking MCP-1, MIP-1α, TNF-α, and GM-CSF together almost completely reduced the number of Mac-1+ macrophages induced by LPC. Mean ± SEM (*p < 0.001);n = 4 animals. Ab, Antibodies.
Fig. 4.
Neutralizing MCP-1, MIP-1α, TNF-α, and GM-CSF together reduces LPC-induced demyelination in the mouse spinal cord.A, B, Light micrographs of toluidine blue-stained Epon-embedded cross-sections of the spinal cord through the region of the dorsal columns, 4 d after injection of LPC plus either control antibodies (A) or all four blocking antibodies (B). In the control antibody-treated animal, LPC produces a wide area of demyelination (A). The size of this area is substantially reduced in mice treated with blocking antibodies (B). Arrows point to the injection sites. Scale bar, 25 μm. C, D, Quantification of the area of demyelination (C) and the amount of myelin present in a 15,000 μm2area on either side of the injection site (D) 4 d after injections of LPC plus either neutralizing antibodies (▪) or control antibodies (■). Measurements were obtained from Epon-embedded sections of the mouse dorsal column. Neutralizing antibodies reduced the area of demyelination induced by LPC by approximately sixfold (C). In addition, myelin clearance within this area was reduced approximately four times in the antibody-treated mice compared with controls (D). Mean ± SEM (*p < 0.008, C;p < 0.002, D);n = 3 animals. Ab, Antibodies.
Similar articles
- T cells contribute to lysophosphatidylcholine-induced macrophage activation and demyelination in the CNS.
Ghasemlou N, Jeong SY, Lacroix S, David S. Ghasemlou N, et al. Glia. 2007 Feb;55(3):294-302. doi: 10.1002/glia.20449. Glia. 2007. PMID: 17096403 - Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration.
Perrin FE, Lacroix S, Avilés-Trigueros M, David S. Perrin FE, et al. Brain. 2005 Apr;128(Pt 4):854-66. doi: 10.1093/brain/awh407. Epub 2005 Feb 2. Brain. 2005. PMID: 15689362 - G-CSF therapy of ongoing experimental allergic encephalomyelitis via chemokine- and cytokine-based immune deviation.
Zavala F, Abad S, Ezine S, Taupin V, Masson A, Bach JF. Zavala F, et al. J Immunol. 2002 Feb 15;168(4):2011-9. doi: 10.4049/jimmunol.168.4.2011. J Immunol. 2002. PMID: 11823538 - Myelin as an inflammatory mediator: Myelin interactions with complement, macrophages, and microglia in spinal cord injury.
Kopper TJ, Gensel JC. Kopper TJ, et al. J Neurosci Res. 2018 Jun;96(6):969-977. doi: 10.1002/jnr.24114. Epub 2017 Jul 11. J Neurosci Res. 2018. PMID: 28696010 Free PMC article. Review.
Cited by
- Role of immune cells in animal models for inherited neuropathies: facts and visions.
Mäurer M, Kobsar I, Berghoff M, Schmid CD, Carenini S, Martini R. Mäurer M, et al. J Anat. 2002 Apr;200(4):405-14. doi: 10.1046/j.1469-7580.2002.00045.x. J Anat. 2002. PMID: 12090406 Free PMC article. Review. - Lysophosphatidic acid-3 receptor-mediated feed-forward production of lysophosphatidic acid: an initiator of nerve injury-induced neuropathic pain.
Ma L, Uchida H, Nagai J, Inoue M, Chun J, Aoki J, Ueda H. Ma L, et al. Mol Pain. 2009 Nov 13;5:64. doi: 10.1186/1744-8069-5-64. Mol Pain. 2009. PMID: 19912636 Free PMC article. - Reelin controls progenitor cell migration in the healthy and pathological adult mouse brain.
Courtès S, Vernerey J, Pujadas L, Magalon K, Cremer H, Soriano E, Durbec P, Cayre M. Courtès S, et al. PLoS One. 2011;6(5):e20430. doi: 10.1371/journal.pone.0020430. Epub 2011 May 27. PLoS One. 2011. PMID: 21647369 Free PMC article. - Endothelial Cells Exposed to Fluid Shear Stress Support Diffusion Based Maturation of Adult Neural Progenitor Cells.
Dumont CM, Piselli J, Temple S, Dai G, Thompson DM. Dumont CM, et al. Cell Mol Bioeng. 2017 Dec 1;11(2):117-130. doi: 10.1007/s12195-017-0516-5. eCollection 2018 Apr. Cell Mol Bioeng. 2017. PMID: 31719881 Free PMC article. - Role of secretory phospholipase a(2) in CNS inflammation: implications in traumatic spinal cord injury.
Titsworth WL, Liu NK, Xu XM. Titsworth WL, et al. CNS Neurol Disord Drug Targets. 2008 Jun;7(3):254-69. doi: 10.2174/187152708784936671. CNS Neurol Disord Drug Targets. 2008. PMID: 18673210 Free PMC article. Review.
References
- Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999;22:504–512. - PubMed
- Bahr M, Przyrembel C. Myelin from peripheral and central nervous system is a non-permissive substrate for retinal ganglion cell axons. Exp Neurol. 1995;134:87–93. - PubMed
- Bandtlow CE, Schwab ME. NI-35/250/Nogo-A: a neurite growth inhibitor restricting structural plasticity and regeneration of nerve fibers in the adult vertebrate CNS. Glia. 2000;29:175–181. - PubMed
- Bartholdi D, Schwab ME. Expression of pro-inflammatory cytokines and chemokines mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci. 1997;9:1422–1438. - PubMed
- Beuche W, Friede RL. The role of non-resident cells in Wallerian degeneration. J Neurocytol. 1984;13:767–796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous