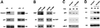Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation - PubMed (original) (raw)
. 2001 Jul 2;20(13):3427-36.
doi: 10.1093/emboj/20.13.3427.
C Talora, R Okuyama, M Nicolas, C Mammucari, H Oh, J C Aster, S Krishna, D Metzger, P Chambon, L Miele, M Aguet, F Radtke, G P Dotto
Affiliations
- PMID: 11432830
- PMCID: PMC125257
- DOI: 10.1093/emboj/20.13.3427
Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation
A Rangarajan et al. EMBO J. 2001.
Abstract
The role of Notch signaling in growth/differentiation control of mammalian epithelial cells is still poorly defined. We show that keratinocyte-specific deletion of the Notch1 gene results in marked epidermal hyperplasia and deregulated expression of multiple differentiation markers. In differentiating primary keratinocytes in vitro endogenous Notch1 is required for induction of p21WAF1/Cip1 expression, and activated Notch1 causes growth suppression by inducing p21WAF1/Cip1 expression. Activated Notch1 also induces expression of 'early' differentiation markers, while suppressing the late markers. Induction of p21WAF1/Cip1 expression and early differentiation markers occur through two different mechanisms. The RBP-Jkappa protein binds directly to the endogenous p21 promoter and p21 expression is induced specifically by activated Notch1 through RBP-Jkappa-dependent transcription. Expression of early differentiation markers is RBP-Jkappa-independent and can be induced by both activated Notch1 and Notch2, as well as the highly conserved ankyrin repeat domain of the Notch1 cytoplasmic region. Thus, Notch signaling triggers two distinct pathways leading to keratinocyte growth arrest and differentiation.
Figures
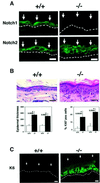
Fig. 1. Inducible keratinocyte-specific deletion of the Notch1 gene and resulting epidermal hyperplasia. (A) Immunofluorescence analysis of frozen skin sections from a mouse with an induced deletion of the Notch1 gene (–/–) and littermate control (+/+) with antibodies against the Notch1 and Notch2 proteins. Identical exposure and image capture conditions were used for comparison of the Notch1+/+ and Notch1–/– skins. Images are representative of multiple fields and similar results were obtained in a second independent experiment. Bar: 10 µm. (B) Hematoxylin and eosin staining of the skin of a mouse with an induced deletion of the Notch1 gene (–/–) and littermate control (+/+). Bar: 15 µm. Lower panels show the quantification of epidermal thickness (left) and cells positive by immunohistochemical staining for the Ki67 proliferation marker (right) in two independent sets of control and induced Notch1–/– littermates. Similar results were obtained with a third set of mice. Same regions of mouse back skin were analyzed in each case. Digitally acquired images from a minimum of three independent areas per section were quantified using a computer-assisted program. The observed differences were highly significant by Fisher test; P values are as indicated. (C) Immunofluorescence analysis of frozen skin sections from a mouse with an induced deletion of the Notch1 gene (–/–) and littermate control (+/+) with antibodies against the hyper-proliferative marker keratin 6. Bar: 8 µm.
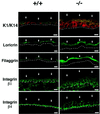
Fig. 2. Aberrant keratinocyte differentiation marker and integrin expression in the epidermis of mice after induced Notch1 deletion. Frozen skin sections from mice with an induced deletion of the Notch1 gene (–/–) and littermate controls (+/+) were analyzed by immunofluorescence with polyclonal antibodies against the indicated proteins and fluorescein isothiocyanate (FITC)-conjugated secondaries. Double staining of keratin 1 and keratin 14 was carried out by sequential incubation with anti-keratin 14 antibodies followed by biotin-conjugated secondaries and Texas red-conjugated streptavidin, and directly FITC-conjugated antibodies against keratin 1. Identical exposure and image capture conditions were used for comparison of the Notch1+/+ and Notch1–/– skins. Similar results were obtained with two and in some cases three independent sets of mice. Bar: 8 µm.
Fig. 3. Increased endogenous Notch activity in differentiating keratinocytes and suppressing effects of a Notch ligand competing peptide. (A) Activation of HES-AB promoter activity by activated Notch1. Primary mouse keratinocytes were transfected with luciferase reporter plasmids carrying the HES-AB promoter or the mutated HES-ΔAB promoter with two site-specific deletions that abrogate RBP-Jκ/Notch-dependent transcription (Jarriault et al., 1995), with or without an expression plasmid expressing the activated form of Notch1 (Capobianco et al., 1997). These and all other promoter activity studies are representative of at least three independent experiments. (B) HES-AB promoter activity in growing versus differentiating keratinocytes. Keratinocytes were transfected with the HES-AB or HES-ΔAB reporters, ± high calcium exposure for the last 24 or 48 h of the experiment (72 h after transfection). (C) Reduced activity of HES-AB promoter by anti-sense Notch1 cDNA. Keratinocytes were transfected with the HES-AB reporter with or without an expression plasmid for anti-sense mouse Notch1 cDNA (nucleotides 1–6805 inserted in the reverse orientation in pcDNA3) (AS-Notch1). Cells were either kept under low calcium conditions or exposed to high calcium concentrations for the last 24 h of the experiment. (D) Insensitivity of the SV40 minimal promoter to inhibition by anti-sense Notch1 cDNA. Same experiment as described in (C), except that keratinocytes were transfected with a reporter plasmid carrying a minimal SV40 early promoter. (E) Increased HES-AB promoter activity in keratinocytes induced to differentiate in suspension, and suppressing effects of a Notch ligand competing peptide (Hurn1EGF11–12 peptide). Keratinocytes were transfected with the HES-AB or HES-ΔAB reporters, and either kept under attached growing conditions (A) or induced to differentiate by detachment from the dish (D) for 24 h ± pretreatment with the Hurn1EGF11–12 peptide. (F) Effects of the Hurn1EGF11–12 peptide on keratinocyte differentiation marker expression. Keratinocytes were induced to differentiate by suspension culture (left panel) or high calcium exposure (right panel). Cells received no other treatment (lane 1), or were pretreated with the Hurn1EGF11–12 peptide (lane 2), or mock pretreated (lane 3). Total cell extracts were analyzed by immunoblotting with antibodies against involucrin and keratin 1. The same blots were stripped and sequentially re-probed with antibodies against keratin 5 and ras-GAP as a measure of equal loading conditions. Densitometric quantification of the autoradiographs showed that, relative to the mock-treated controls, involucrin expression was suppressed ∼3-fold by the Hurn1EGF11–12 peptide in both calcium-treated cells and cells brought into suspension. Keratin 1 expression was suppressed ∼10-fold by the Hurn1EGF11–12 peptide in the calcium-treated cells, and ∼3-fold in the cells brought into suspension. Similar results were obtained in two independent experiments.
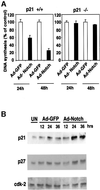
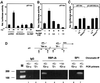
Fig. 4. Growth suppression of keratinocytes by activated Notch1 through a p21_WAF1/Cip1_-dependent mechanism. (A) DNA synthesis in wild-type and p21-null keratinocytes infected with a control GFP adenovirus (Ad-GFP) or an adenovirus expressing the constitutively active form of Notch1 (Ad-Notch). Primary keratinocytes derived from wild-type mice (left) or mice with a homozygous disruption of the p21_WAF1/Cip1_ gene in the same genetic background as the controls (right), were pulse-labeled with [3H]thymidine for 1.5 h at the indicated times (h) after adenoviral vector infection, and [3H]thymidine incorporation was determined as described (Missero et al., 1996). All samples were tested in triplicate wells, and standard deviation is indicated. Values are expressed as percentages relative to the adeno-GFP-infected controls. Similar results were observed in two independent experiments. (B) Induction of p21_WAF1/Cip1_ protein expression by activated Notch1 expression. Total cell extracts were prepared from keratinocytes infected with the GFP control or activated Notch adenoviruses at the indicated times (h) after infection. UN: uninfected control. The same protein amounts (30 µg) were analyzed by 12.5% SDS–PAGE and immunoblotting with monoclonal antibodies against mouse p21_WAF1/Cip1_ or p27_Kip1_ proteins. The same blot was stripped and reprobed with anti-CDK2 antibodies. Densitometric quantification of the autoradiographs indicated that p21 expression was induced ∼4-fold in cells infected with the Ad-Notch1 adenovirus versus the Ad-GFP control at both 24 and 36 h after infection. A similar induction of p21 protein expression was observed in two other independent experiments.
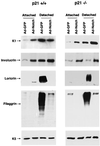
Fig. 5. Induction of early differentiation markers and suppression of late markers by activated Notch1. Primary keratinocytes from wild-type (left) or p21 null mice (right) with the same genetic background were infected under low calcium conditions with the GFP control or activated Notch1 adenoviruses, and total cell extracts were collected from the attached and spontaneously detached cell populations at 36 h after infection. The same protein amounts (10 µg) were analyzed by 7.5% SDS–PAGE and immunoblotting with antibodies against the various differentiation markers as previously reported (Missero et al., 1996). Filaggrin is synthesized as a high molecular weight precursor, profilaggrin, which is subsequently processed. The diffuse bands correspond to the multiple products of this processing. The same blots were stripped and reprobed with anti-keratin 5 antibodies as control for equal loading conditions. Similar results were obtained in three independent experiments.
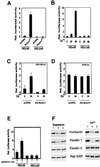
Fig. 6. Induction of p21 expression by activated Notch1 through RBP-Jκ-dependent transcription. (A) Suppression of p21 promoter activity in differentiating keratinocytes by antisense Notch1 cDNA and dominant-negative RBP-Jκ. Keratinocytes were transfected with a reporter plasmid carrying the proximal 2.4 kb region of the human p21 promoter (p21-luc) ± expression vectors for anti-sense mouse Notch1 cDNA or dominant-negative RBP-Jκ (Kato et al., 1997). Cells were kept in low calcium medium or exposed to high calcium for the last 24 h of the experiment (72 h after transfection). (B) RBP-Jκ-dependent induction of p21 promoter activity by activated Notch1. Keratinocytes were transfected with the p21-luc reporter ± an expression vector for activated Notch1 (Notch1ICD) and a vector for the dominant-negative RBP-Jκ mutant in increasing amounts. Empty plasmid vector was also added to ensure that all cells were transfected with the same amount of total DNA. (C) Calcium and Notch1 responsiveness of a minimal p21 promoter region containing the fully conserved RBP-Jκ binding site. Keratinocytes were transfected with the reporters p21-luc, carrying the 2.4 kb p21 promoter, or p21-(557/382)-luc, containing 175 bp of the p21 promoter containing the RBP-Jκ binding site (–557 to –382 position) fused to a minimal promoter (pluc-MCS vector; Stratagene). Cells were either kept under low calcium conditions, exposed to high calcium for 24 h or co-transfected with an expression vector for activated Notch1. (D) Binding of the RBP-Jκ protein to the endogenous p21 promoter as assessed by chromatin immunoprecipitation. Primary keratinocytes under low calcium conditions were processed for chromatin immunoprecipitation with antibodies against the RBP-Jκ or SP1 proteins and affinity-purified IgGs. The immunoprecipitates were analyzed by PCR with oligonucleotide primers specific for the indicated regions of the mouse p21_WAF1/Cip1_ promoter and for a region of the mouse c-jun promoter containing a canonical RBP-Jκ binding site. Preliminary PCR reactions were carried out with naked DNA to determine optimal conditions for amplification of each DNA.
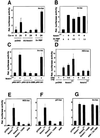
Fig. 7. Induction of involucrin expression by activated Notch1 and Notch2 expression and the conserved Notch1 ANK domain. (A) Suppression of involucrin promoter activity in differentiating keratinocytes by expression of anti-sense Notch1 cDNA but not dominant-negative RBP-Jκ. Keratinocytes were transfected with a reporter plasmid (Inv-luc) carrying the human involucrin promoter (Brissette et al., 1996) ± expression vectors for anti-sense mouse Notch1 cDNA or dominant-negative RBP-Jκ. Cells were kept under low calcium conditions or exposed to high calcium for 24 h. (B) Induction of involucrin promoter activity by activated Notch1 independently of RBP-Jκ activity. Keratinocytes were transfected with the Inv-luc reporter ± an expression vector for activated Notch1 (Notch1ICD) and a vector for dominant-negative RBP-Jκ in increasing amounts. Empty plasmid vector was also added to ensure that all cells were transfected with the same amount of total DNA. (C) Identification of the minimal Notch responsive region of involucrin promoter. Primary keratinocytes were transfected with reporter plasmids carrying either 2473, 2116–2088 or 241 bases (Efimova et al., 1998) of the involucrin promoter region, ± an expression vector for activated Notch1 cDNA. (D) Differential induction of the RBP-Jκ responsive HES-AB promoter by activated Notch1 versus Notch2 expression. Keratinocytes were transfected with the HES-AB reporter with increasing amounts of expression vectors for either activated Notch1 or Notch2 proteins (Capobianco et al., 1997). An analogous dose–response experiment was also performed with the involucrin promoter (as in G), which, unlike the HES-AB promoter, showed similar induction by both activated Notch1 and Notch2 (not shown). (E–G) Selective induction of involucrin promoter activity by activated Notch2 and the conserved Notch1 ANK domain. Keratinocytes were transfected with reporter plasmids for the HES-AB (E), p21 (F) and involucrin (G) promoters with or without expression plasmids for the activated cytoplasmic region of Notch1 (Notch1ICD), the same region with an internal deletion of the ANK domain (Notch1-ΔANK), the Notch1 ANK domain alone (ANK) and activated Notch2 (Notch2ICD). The Notch1 ANK domain was also co-expressed with increasing amounts of the vector for dominant-negative RBP-Jκ as in (B); shown is the result with 1.5 µg of transfected plasmid DNA for the mutant RBP-Jκ.
Fig. 8. Separate regulation of endogenous p21 and differentiation markers by Notch signaling. (A) p21 and differentiation marker expression in cultured keratinocytes with induced deletion of the Notch1 gene. Primary keratinocytes derived from mice homozygous for the Notch1/flox gene were infected with GFP- or Cre-expressing adenoviruses and, 4 days after infection, were induced to differentiate by high calcium exposure. Total cell extracts from cells with (+/+) or without the Notch1 gene (–/–) were analyzed by immunoblotting with antibodies against the indicated proteins. Similar results were obtained in a second independent experiment. (B) RBP-Jκ-independent expression of keratinocyte differentiation markers. Keratinocytes were transfected with an expression vector for GFP together with an expression vector for dominant-negative RBP-Jκ or empty vector control. Forty-eight hours after transfection, cells were induced to differentiate by suspension culture as previously described (Di Cunto et al., 1998). Transfected GFP-positive cells were purified by sorting and analyzed by sequential immunoblotting with antibodies against the indicated proteins, including ras-GAP as an unrelated internal control. (C and D) Keratinocytes under growing low calcium conditions were infected with a GFP control adenovirus or an adenovirus expressing the ANK domain of Notch1 (Ad-ANK) for either 24 or 36 h as indicated. Total cell extracts were analyzed by SDS–PAGE and sequential immunoblotting with antibodies against the indicated cell cycle regulatory proteins (C) or differentiation markers (D).
Similar articles
- Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control.
Mammucari C, Tommasi di Vignano A, Sharov AA, Neilson J, Havrda MC, Roop DR, Botchkarev VA, Crabtree GR, Dotto GP. Mammucari C, et al. Dev Cell. 2005 May;8(5):665-76. doi: 10.1016/j.devcel.2005.02.016. Dev Cell. 2005. PMID: 15866158 - p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation.
Devgan V, Mammucari C, Millar SE, Brisken C, Dotto GP. Devgan V, et al. Genes Dev. 2005 Jun 15;19(12):1485-95. doi: 10.1101/gad.341405. Genes Dev. 2005. PMID: 15964998 Free PMC article. - A dynamic model of keratinocyte stem cell renewal and differentiation: role of the p21WAF1/Cip1 and Notch1 signaling pathways.
Okuyama R, LeFort K, Dotto GP. Okuyama R, et al. J Investig Dermatol Symp Proc. 2004 Sep;9(3):248-52. doi: 10.1111/j.1087-0024.2004.09308.x. J Investig Dermatol Symp Proc. 2004. PMID: 15369220 Review. - Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation.
Talora C, Sgroi DC, Crum CP, Dotto GP. Talora C, et al. Genes Dev. 2002 Sep 1;16(17):2252-63. doi: 10.1101/gad.988902. Genes Dev. 2002. PMID: 12208848 Free PMC article. - Signal transduction pathways controlling the switch between keratinocyte growth and differentiation.
Dotto GP. Dotto GP. Crit Rev Oral Biol Med. 1999;10(4):442-57. doi: 10.1177/10454411990100040201. Crit Rev Oral Biol Med. 1999. PMID: 10634582 Review.
Cited by
- Does Notch play a tumor suppressor role across diverse squamous cell carcinomas?
Zhang M, Biswas S, Qin X, Gong W, Deng W, Yu H. Zhang M, et al. Cancer Med. 2016 Aug;5(8):2048-60. doi: 10.1002/cam4.731. Epub 2016 May 26. Cancer Med. 2016. PMID: 27228302 Free PMC article. Review. - De Novo Transcriptome Assembly and Differential Gene Expression Profiling of Three Capra hircus Skin Types during Anagen of the Hair Growth Cycle.
Xu T, Guo X, Wang H, Du X, Gao X, Liu D. Xu T, et al. Int J Genomics. 2013;2013:269191. doi: 10.1155/2013/269191. Epub 2013 May 20. Int J Genomics. 2013. PMID: 23762818 Free PMC article. - Top Notch Targeting Strategies in Cancer: A Detailed Overview of Recent Insights and Current Perspectives.
Moore G, Annett S, McClements L, Robson T. Moore G, et al. Cells. 2020 Jun 20;9(6):1503. doi: 10.3390/cells9061503. Cells. 2020. PMID: 32575680 Free PMC article. Review. - Cutaneous β-human papillomavirus E6 proteins bind Mastermind-like coactivators and repress Notch signaling.
Tan MJ, White EA, Sowa ME, Harper JW, Aster JC, Howley PM. Tan MJ, et al. Proc Natl Acad Sci U S A. 2012 Jun 5;109(23):E1473-80. doi: 10.1073/pnas.1205991109. Epub 2012 Apr 30. Proc Natl Acad Sci U S A. 2012. PMID: 22547818 Free PMC article. - The functional role of Notch signaling in human gliomas.
Stockhausen MT, Kristoffersen K, Poulsen HS. Stockhausen MT, et al. Neuro Oncol. 2010 Feb;12(2):199-211. doi: 10.1093/neuonc/nop022. Epub 2009 Dec 14. Neuro Oncol. 2010. PMID: 20150387 Free PMC article. Review.
References
- Artavanis-Tsakonas S., Rand,M.D. and Lake,R.J. (1999) Notch signaling: cell fate control and signal integration in development. Science, 284, 770–776. - PubMed
- Aster J.C., Robertson,E.S., Hasserjian,R.P., Turner,J.R., Kieff,E. and Sklar,J. (1997) Oncogenic forms of NOTCH1 lacking either the primary binding site for RBP-Jκ or nuclear localization sequences retain the ability to associate with RBP-Jκ and activate transcription. J. Biol. Chem., 272, 11336–11343. - PubMed
- Brissette J., Li,J., Kamimura,J., Lee,D. and Dotto,G.P. (1996) The product of the mouse nude locus, Whn, regulates the balance between epithelial cell growth and differentiation. Genes Dev., 10, 2212–2221. - PubMed
- Carroll J.M., Romero,M.R. and Watt,F.M. (1995) Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell, 83, 957–968. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR39190/AR/NIAMS NIH HHS/United States
- CA16038/CA/NCI NIH HHS/United States
- R01 CA073796/CA/NCI NIH HHS/United States
- R01 AR039190/AR/NIAMS NIH HHS/United States
- P01 CA016038/CA/NCI NIH HHS/United States
- CA73796/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous