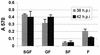Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene - PubMed (original) (raw)
Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene
S Techaarpornkul et al. J Virol. 2001 Aug.
Abstract
Respiratory syncytial virus (RSV) produces three envelope glycoproteins, the attachment glycoprotein (G), the fusion (F) protein, and the small hydrophobic (SH) protein. It had been assumed, by analogy with other paramyxoviruses, that the G and F proteins would be required for the first two steps of viral entry, attachment and fusion. However, following repeated passage in cell culture, a viable mutant RSV that lacked both the G and SH genes was isolated (R. A. Karron, D. A. Buonagurio, A. F. Georgiu, S. S. Whitehead, J. E. Adamus, M. L. Clements-Mann, D. O. Harris, V. B. Randolph, S. A. Udem, B. R. Murphy, and M. S. Sidhu, Proc. Natl. Acad. Sci. USA 94:13,961--13,966, 1997). To explore the roles of the G, F, and SH proteins in virion assembly, function, and cytopathology, we have modified the full-length RSV cDNA and used it to rescue infectious RSV lacking the G and/or SH genes. The three resulting viruses and the parental virus all contain the green fluorescent protein (GFP) gene that serves to identify infected cells. We have used purified, radiolabeled virions to examine virus production and function, in conjunction with GFP to quantify infected cells. We found that the G protein enhances virion binding to target cells but plays no role in penetration after attachment. The G protein also enhances cell-to-cell fusion, presumably via cell-to-cell binding, and enhances virion assembly or release. The presence or absence of the G protein in virions has no obvious effect on the content of F protein or host cell proteins in the virion. In growth curve experiments, the viruses lacking the G protein produced viral titers that were at least 10-fold lower than titers of viruses containing the G protein. This reduction is due in large part to the less efficient release of virions and the lower infectivity of the released virions. In the absence of the G protein, virus expressing both the F and SH proteins displayed somewhat smaller plaques, lower fusion activity, and slower viral entry than the virus expressing the F protein alone, suggesting that the SH protein has a negative effect on virus fusion in cell culture.
Figures
FIG. 1
Diagram of the SN3 plasmid and its three derivative plasmids, SN10, SN11, and SN12 (not to scale). SN3 is a partial RSV antigenome plasmid, containing all of the RSV genes except for the three viral glycoprotein genes. Like its complete RSV parent cDNA, MP224, SN3 contains the GFP gene in the first position. The new intergenic sequence (IG) between the M and M2 genes contains two unique restriction sites, _Pvu_I and _Xho_I, as well as native IG sequence before and after these sites, as indicated. These sites were used to subclone RSV genes back into SN3: the G and F genes to generate SN11, and the F gene to generate SN10. Le, leader; TR, trailer; nt, nucleotides. SN11 and SN10 were used to rescue rgRSV-GF and rgRSV-F, respectively. The SH gene was subcloned into the _Pvu_I and _Sac_II sites of SN10 to generate SN12, which was used to rescue rgRSV-SF. All inserted genes (large open rectangles) and gene start and gene end sequences (shaded boxes) are authentic. The sequence and derivation of all modified IG regions are shown, including the only nonnative sequences and the added restriction sites (bold italics). Transcription from all plasmids was driven by the T7 promoter (indicated on SN3) to initiate synthesis of the antigenome.
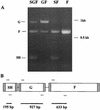
FIG. 2
RT-PCR verification of the glycoprotein genes present in each rescued virus. (A) RT-PCR products from RNA extracted from purified rgRSV-SGF, rgRSV-GF, rgRSV-SF, and rgRSV-F virions. The PCR products were separated by electrophoresis on a 1.5% agarose gel and stained with ethidium bromide. (B) Three sets of PCR primers (arrows) were used to amplify segments of the SH, G, and F genes in one reaction tube for each virus.
FIG. 3
Protein expression in virus-infected cells and in purified virions. (A) HEp-2 cells mock infected or infected with rgRSV-SGF, rgRSV-GF, rgRSV-SF, or rgRSV-F were labeled with [35S]Met-[35S]Cys. Lysates prepared from these cells were immunoprecipitated with rabbit anti-RSV serum, reduced, and separated by SDS-PAGE. (B) [35S]Met-[35S]Cys virions were purified twice by equilibrium centrifugation; 30,000 cpm of each virus was reduced and electrophoresed in the same gel as in panel A. The RSV proteins are indicated on the right, and molecular mass markers (kilodaltons) are shown on the left.
FIG. 4
Growth curves for rgRSV-SGF, rgRSV-GF, rgRSV-SF, and rgRSV-F. HEp-2 cells were inoculated (MOI ∼ 1) and incubated at 37°C. At the indicated times, samples of culture medium were removed and frozen at −70°C. Virus was titrated at the same time in duplicate; the results averaged and reported as infectious units (IU) per milliliter. The experiment was repeated a total of three times with similar results, a 1- to 2-log-lower titer for rgRSV-SF and rgRSV-F compared to rgRSV-SGF and rgRSV-GF.
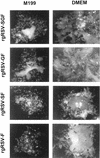
FIG. 5
Typical plaques produced by rgRSV-SGF, rgRSV-GF, rgRSV-SF, and rgRSV-F in different overlay media. HEp-2 cells were inoculated, overlaid with 0.8% agarose in medium 199 or DMEM, and incubated at 37°C for 6 days. Plaques were photographed with a 10× objective.
FIG. 6
Release of virus particles from HEp-2 cells infected with rgRSV-SGF, rgRSV-GF, rgRSV-SF, and rgRSV-F. Monolayers (two 150-mm-diameter plates each) were inoculated (MOI ∼ 2) and incubated at 37°C. At 24 h, approximately the same number of cells were infected (85% green) in all plates. Cells were labeled with [35S]Met-[35S]Cys from 30 to 54 h. Culture media were harvested and clarified by low-speed centrifugation. Virions were purified by equilibrium centrifugation through two sequential linear sucrose gradients. The counts per minute (CPM) plotted is the sum from the peak fractions of the second gradient.
FIG. 7
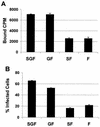
Binding and infectivity of purified rgRSV-SGF, rgRSV-GF, rgRSV-SF, and rgRSV-F virions. Twice-sucrose gradient-purified [35S]Met-[35S]Cys virions (30,000 cpm) were incubated with confluent monolayers of HEp-2 cells at 4°C for 1 h and then washed four times with ice-cold medium to remove unbound virus. (A) To assess binding, cells were lysed and radioactivity was quantified by a beta counter. The average background from wells containing only medium was subtracted (∼100 cpm). (B) To assess infectivity, growth medium was added and cells were incubated at 37°C for 18 h. The percent infected (green) cells was determined by flow cytometry. The results are the average ± standard deviation of three wells. The two assays were performed at the same time. These data are representative of three separate experiments.
FIG. 8
Postattachment entry rate of rgRSV-SGF, rgRSV-GF, rgRSV-SF, and rgRSV-F virions. Each virus (MOI < 1) was adsorbed to a confluent monolayer of HEp-2 cells at 4°C for 1 h. Cells were rinsed to remove unbound virus and moved to 37°C to allow penetration. At the indicated times, cells were treated with pH 3 citrate-buffered saline for 1 min to inactivate viruses remaining on the cell surface and rinsed, OPTI-MEM–2% FBS was added, and cells were incubated at 37°C. PBS instead of citrate-buffered saline was applied to control, nontreated cells. After incubation at 37°C for 15 h, infected (green) cells were counted by flow cytometry. Wells that had been treated with the pH 3 buffer before shifting to 37°C resulted in no infected (green) cells. Percent entry was calculated by dividing the percent infected cells in the pH 3-treated wells at each time by the percent infected cells in the parallel PBS-treated well and multiplying by 100. This experiment represents three separate experiments with similar results.
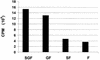
FIG. 9
Fusogenic activity of rgRSV-SGF, rgRSV-GF, rgRSV-SF, and rgRSV-F. HEp-2 cell monolayers were inoculated with each virus (MOI ∼2) and then with vT7lacOI encoding T7 polymerase. At 36 or 42 h p.i., these cells were overlaid with a second population of HEp-2 cells infected with vCB21RlacZ, carrying a β-galactosidase gene preceded by a T7 promoter. Fusion of these two cell types allows the T7 polymerase to drive expression of the β-galactosidase gene. After 3 h at 37°C, fusion was quantified by measuring β-galactosidase activity with the colorimetric CRPG assay. The infection level of each monolayer was assessed (65 to 80% green cells), and fusion values were normalized to account for these small differences. The mean values of three wells ± standard deviations are shown. These data are representative of two separate experiments with similar results.
Similar articles
- The RSV fusion receptor: not what everyone expected it to be.
Mastrangelo P, Hegele RG. Mastrangelo P, et al. Microbes Infect. 2012 Nov;14(13):1205-10. doi: 10.1016/j.micinf.2012.07.015. Epub 2012 Jul 31. Microbes Infect. 2012. PMID: 22884716 Review. - The Role of Phlebovirus Glycoproteins in Viral Entry, Assembly and Release.
Spiegel M, Plegge T, Pöhlmann S. Spiegel M, et al. Viruses. 2016 Jul 21;8(7):202. doi: 10.3390/v8070202. Viruses. 2016. PMID: 27455305 Free PMC article. Review.
Cited by
- Protein-Protein Interactions of Viroporins in Coronaviruses and Paramyxoviruses: New Targets for Antivirals?
Torres J, Surya W, Li Y, Liu DX. Torres J, et al. Viruses. 2015 Jun 4;7(6):2858-83. doi: 10.3390/v7062750. Viruses. 2015. PMID: 26053927 Free PMC article. Review. - Respiratory syncytial virus-neutralizing monoclonal antibodies motavizumab and palivizumab inhibit fusion.
Huang K, Incognito L, Cheng X, Ulbrandt ND, Wu H. Huang K, et al. J Virol. 2010 Aug;84(16):8132-40. doi: 10.1128/JVI.02699-09. Epub 2010 Jun 2. J Virol. 2010. PMID: 20519399 Free PMC article. - Respiratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV.
Johnson TR, Teng MN, Collins PL, Graham BS. Johnson TR, et al. J Virol. 2004 Jun;78(11):6024-32. doi: 10.1128/JVI.78.11.6024-6032.2004. J Virol. 2004. PMID: 15141000 Free PMC article. - Recombinant respiratory syncytial virus F protein expression is hindered by inefficient nuclear export and mRNA processing.
Huang K, Lawlor H, Tang R, MacGill RS, Ulbrandt ND, Wu H. Huang K, et al. Virus Genes. 2010 Apr;40(2):212-21. doi: 10.1007/s11262-010-0449-8. Epub 2010 Jan 29. Virus Genes. 2010. PMID: 20111897 - Cationic-nanogel nasal vaccine containing the ectodomain of RSV-small hydrophobic protein induces protective immunity in rodents.
Umemoto S, Nakahashi-Ouchida R, Yuki Y, Kurokawa S, Machita T, Uchida Y, Mori H, Yamanoue T, Shibata T, Sawada SI, Ishige K, Hirano T, Fujihashi K, Akiyoshi K, Kurashima Y, Tokuhara D, Ernst PB, Suzuki M, Kiyono H. Umemoto S, et al. NPJ Vaccines. 2023 Jul 24;8(1):106. doi: 10.1038/s41541-023-00700-3. NPJ Vaccines. 2023. PMID: 37488116 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources