A novel p75NTR signaling pathway promotes survival, not death, of immunopurified neocortical subplate neurons - PubMed (original) (raw)
A novel p75NTR signaling pathway promotes survival, not death, of immunopurified neocortical subplate neurons
M F DeFreitas et al. J Neurosci. 2001.
Abstract
Subplate neurons of mammalian neocortex undergo pronounced cell death postnatally, long after they have matured and become incorporated into functional cortical circuits. They express the p75 neurotrophin receptor (p75NTR), which is known to signal cell death in some types of neurons via the activation of sphingomyelinase and the concomitant increase in the sphingolipid ceramide. To evaluate the role of p75NTR in subplate neurons, they were immunopurified and cultured in vitro. Contrary to its known function as a death receptor, ligand binding to p75NTR promotes subplate neuron survival. Moreover, p75NTR-dependent survival is blocked by inhibition of ceramide synthesis and rescued by addition of its precursor sphingomyelin. Inhibition of Trk signaling does not block survival, nor is Trk signaling alone sufficient to promote survival. Thus, ligand-dependent p75NTR regulation of the ceramide pathway mediates survival in certain neurons and may represent an important target for neuroprotective drugs in degenerative diseases involving p75NTR-expressing neurons, such as Alzheimer's disease.
Figures
Fig. 1.
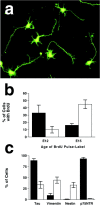
Subplate neurons, but not neurons of the cortical plate, express p75NTR. Localization of mRNA for p75NTR, TrkA, TrkB, and TrkC on parasagittal sections of E17 rat brain. For localization of the subplate neurons, comparable sections were stained with cresyl violet or with an antibody to BrdU. BrdU was given at E12 to label earliest-generated subplate and marginal zone neurons. Heaviest BrdU-labeled cells are restricted to marginal zone and the subplate. Note that subplate neurons are the only p75NTR-expressing neurons at this age. m, Meninges; MZ, marginal zone; CP, cortical plate; SP, subplate. Scale bar, 100 μm.
Fig. 2.
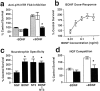
Immunopurification of subplate neurons.a, p75NTR-immunopanned cells grown in culture 4 d, stained with calcein-AM, and visualized by fluorescence microscopy.b, Birth dating of immunopanned cells (black bars) and unbound cells (white bars). A BrdU pulse was given at E12 (birth date of subplate neurons) or E15 (birth date of neurons in layers V and VI), and the percentage of cells labeled with BrdU at E17 was determined. c, Percentage of panned cells (black bars) and unbound cells (white bars) immunostained for Tau, vimentin, nestin, or p75NTR. Results are mean ± SEM.
Fig. 3.
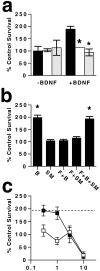
Neurotrophin-mediated survival of subplate neurons is dependent on p75NTR. a, Survival of subplate neurons in the absence or presence of 30 ng/ml exogenous BDNF. Anti-p75NTR FAbs (black bars) or normal rabbit FAbs (white bars) were added and compared with survival in the absence of FAbs (shaded bars). *p < 0.01 compared with BDNF with no FAbs. b, Dose–response curve for BDNF-dependent survival of subplate neurons.c, Survival of panned subplate neurons in the presence of 100 ng/ml neurotrophins (normalized to neurons grown in the absence of exogenous neurotrophin). *p < 0.01 compared with control. d, Subplate neurons were cultured with BDNF (3 ng/ml) with (black bars) or without (white bars) excess NGF (3 μg/ml). *p = 0.016 compared with BDNF alone.
Fig. 4.
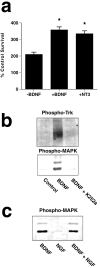
Trk signaling is not necessary for BDNF-dependent survival. a, Survival of subplate neurons grown in K252a in the absence or presence of 100 ng/ml neurotrophins. Data are normalized to survival in the absence of exogenous neurotrophin or K252a. *p < 0.01 compared with K252a alone;t test. b, K252a blocks activation of Trks and MAPK. Extracts of subplate neurons grown in the absence of BDNF or in the presence of BDNF with or without K252a were resolved by Western blot and probed with antibodies to all phosphorylated (i.e., active) Trks or MAPK. c, High concentrations of NGF do not activate the MAPK signaling pathway, nor do they block BDNF-dependent activation of MAPK. Western blot of subplate neurons grown in the presence of BDNF, NGF, or both BDNF and NGF and probed with an antibody to phosphorylated MAPK. The concentration of NGF was 3 μg/ml, 1000-fold higher than the concentration of BDNF.
Fig. 5.
Sphingomyelin synthesis is necessary for p75NTR-dependent survival. a, Subplate neurons were cultured with BDNF (black bars) in the presence of fumonisin B1 (white bars) or myriocin (hatched bars). Survival is normalized to that in the absence of additives. *p < 0.01 compared with BDNF alone.b, Survival of subplate neurons grown in the presence of BDNF (B), fumonisin B1 (F), or sphingomyelin (SM) in combinations indicated. *p < 0.01 compared with control survival;t test. c, Dose–response curve for sphingomyelin rescue of BDNF-dependent survival. Subplate neurons were grown in the presence of fumonisin and increasing concentrations of sphingomyelin with BDNF (black squares) or without BDNF (white squares). Dashed line represents survival in BDNF alone.
Similar articles
- A novel role for p75NTR in subplate growth cone complexity and visual thalamocortical innervation.
McQuillen PS, DeFreitas MF, Zada G, Shatz CJ. McQuillen PS, et al. J Neurosci. 2002 May 1;22(9):3580-93. doi: 10.1523/JNEUROSCI.22-09-03580.2002. J Neurosci. 2002. PMID: 11978834 Free PMC article. - Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75.
Culmsee C, Gerling N, Lehmann M, Nikolova-Karakashian M, Prehn JH, Mattson MP, Krieglstein J. Culmsee C, et al. Neuroscience. 2002;115(4):1089-108. doi: 10.1016/s0306-4522(02)00539-0. Neuroscience. 2002. PMID: 12453482 - Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons.
Brann AB, Scott R, Neuberger Y, Abulafia D, Boldin S, Fainzilber M, Futerman AH. Brann AB, et al. J Neurosci. 1999 Oct 1;19(19):8199-206. doi: 10.1523/JNEUROSCI.19-19-08199.1999. J Neurosci. 1999. PMID: 10493721 Free PMC article. - Coupling of the p75 neurotrophin receptor to sphingolipid signaling.
Dobrowsky RT, Carter BD. Dobrowsky RT, et al. Ann N Y Acad Sci. 1998 Jun 19;845:32-45. doi: 10.1111/j.1749-6632.1998.tb09660.x. Ann N Y Acad Sci. 1998. PMID: 9668341 Review. - p75NTR: A study in contrasts.
Barker PA. Barker PA. Cell Death Differ. 1998 May;5(5):346-56. doi: 10.1038/sj.cdd.4400375. Cell Death Differ. 1998. PMID: 10200483 Review.
Cited by
- Neurotrophin Analog ENT-A044 Activates the p75 Neurotrophin Receptor, Regulating Neuronal Survival in a Cell Context-Dependent Manner.
Papadopoulou MA, Rogdakis T, Charou D, Peteinareli M, Ntarntani K, Gravanis A, Chanoumidou K, Charalampopoulos I. Papadopoulou MA, et al. Int J Mol Sci. 2023 Jul 20;24(14):11683. doi: 10.3390/ijms241411683. Int J Mol Sci. 2023. PMID: 37511441 Free PMC article. - Astrocytes in Neural Circuits: Key Factors in Synaptic Regulation and Potential Targets for Neurodevelopmental Disorders.
Liu X, Ying J, Wang X, Zheng Q, Zhao T, Yoon S, Yu W, Yang D, Fang Y, Hua F. Liu X, et al. Front Mol Neurosci. 2021 Sep 29;14:729273. doi: 10.3389/fnmol.2021.729273. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34658786 Free PMC article. Review. - Transient cortical circuits match spontaneous and sensory-driven activity during development.
Molnár Z, Luhmann HJ, Kanold PO. Molnár Z, et al. Science. 2020 Oct 16;370(6514):eabb2153. doi: 10.1126/science.abb2153. Science. 2020. PMID: 33060328 Free PMC article. Review. - Signaling via the p75 neurotrophin receptor facilitates amyloid-β-induced dendritic spine pathology.
Patnaik A, Zagrebelsky M, Korte M, Holz A. Patnaik A, et al. Sci Rep. 2020 Aug 7;10(1):13322. doi: 10.1038/s41598-020-70153-4. Sci Rep. 2020. PMID: 32770070 Free PMC article. - Nerve Growth Factor in Alcohol Use Disorders.
Ceci FM, Ferraguti G, Petrella C, Greco A, Ralli M, Iannitelli A, Carito V, Tirassa P, Chaldakov GN, Messina MP, Ceccanti M, Fiore M. Ceci FM, et al. Curr Neuropharmacol. 2021;19(1):45-60. doi: 10.2174/1570159X18666200429003239. Curr Neuropharmacol. 2021. PMID: 32348226 Free PMC article. Review.
References
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. - PubMed
- Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials