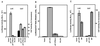Control of cyclin-dependent kinase inhibitor p27 expression by cap-independent translation - PubMed (original) (raw)
Control of cyclin-dependent kinase inhibitor p27 expression by cap-independent translation
W K Miskimins et al. Mol Cell Biol. 2001 Aug.
Abstract
p27 is a key regulator of cell proliferation through inhibition of G(1) cyclin-dependent kinase (CDK) activity. Translation of the p27 mRNA is an important control mechanism for determining cellular levels of the inhibitor. Nearly all eukaryotic mRNAs are translated through a mechanism involving recognition of the 5' cap by eukaryotic initiation factor 4E (eIF4E). In quiescent cells eIF4E activity is repressed, leading to a global decline in translation rates. In contrast, p27 translation is highest during quiescence, suggesting that it escapes the general repression of translational initiation. We show that the 5' untranslated region (5'-UTR) of the p27 mRNA mediates cap-independent translation. This activity is unaffected by conditions in which eIF4E is inhibited. In D6P2T cells, elevated cyclic AMP levels cause a rapid withdrawal from the cell cycle that is correlated with a striking increase in p27. Under these same conditions, cap-independent translation from the p27 5'-UTR is enhanced. These results indicate that regulation of internal initiation of translation is an important determinant of p27 protein levels.
Figures
FIG. 1
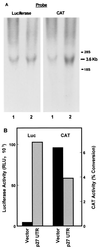
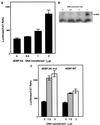
Expression of bicistronic CAT/luciferase constructs in stably transfected NIH 3T3 cells. (A) RNA was isolated from NIH 3T3 cells stably transfected with either pGL2CAT/Luc vector (lane 1) or pGL2CAT/Luc-p27UTR (lane 2). The RNA was analyzed by Northern blotting using the probe indicated. The positions of 28S and 18S rRNAs are indicated at the right. (B) A portion of the same population of cells stably transfected with either pGL2CAT/Luc (vector) or pGL2CAT/Luc-p27UTR (p27 UTR) was harvested and assayed for both luciferase (Luc) and CAT activities.
FIG. 2
Cap-independent translation mediated by the p27 5′-UTR. Bicistronic mRNAs encoding CAT upstream of luciferase were expressed in proliferating D6P2T cells. Cells were transfected with either pGL2CAT/Luc (vector), which has a short spacer region between the CAT and luciferase coding regions, pGL2CAT/Luc-p27UTR, which carries the region encoding the mouse p27 5′-UTR inserted within the spacer region, pGL2CAT/Luc-p27UTR(rev), which carries the region encoding the mouse p27 5′-UTR inserted in reverse orientation within the spacer region, or pGL2CAT/Luc-TR-UTR, which carries 200 bp of the TR 5′-UTR inserted within the spacer region. After cell lysis, CAT and luciferase (LUC) activities were determined for each extract (A), and the relative level of cap-independent translation was estimated from the luciferase/CAT ratio (B). The luciferase/CAT ratio was determined by dividing the relative light units (luciferase) by the percentage of substrate converted to the acetylated form (CAT). (C) D6P2T cells were transfected with either pGL2CAT/Luc (vector), pGL2CAT/Luc-p27UTR, or pcBiP, which carries the BIP 5′-UTR inserted between CAT and luciferase in the plasmid pcDNA1. The cells were harvested 1 day after transfection and assayed for CAT and luciferase activities. All data represent the mean of a minimum of three replicates plus or minus the standard error.
FIG. 3
Cap-independent translation of p27 in the presence of a strong hairpin-loop. NIH 3T3 cells were transfected with the indicated expression constructs (see text for descriptions), harvested after 1 day, and assayed for luciferase activity. All data represent the mean of a minimum of three replicates plus or minus the standard error.
FIG. 4
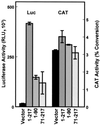
Cap-independent translation mediated by p27 5′-UTR deletion mutants. D6P2T cells were transfected with CAT/luciferase (Luc) constructs containing inserts within the spacer region as indicated. Region 1-217 encodes the entire 5′-UTR (pGL2CAT/Luc-p27UTR), region 71-217 encodes the region immediately proximal to the p27 AUG start codon, and region 1-90 encodes the distal region of the 5′-UTR. All data represent the mean of a minimum of three replicates plus or minus the standard error.
FIG. 5
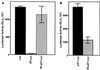
Inhibition of cap-dependent translation does not affect p27 5′-UTR IRES activity. (A) D6P2T cells were transfected with the bicistronic construct carrying the full-length p27 5′-UTR, pGL2CAT/Luc-p27UTR. The cells were then treated with rapamycin (200 ng/ml) as indicated (+Rap). Each extract was analyzed for luciferase (Luc) and CAT activities. All data represent the mean of a minimum of three replicates plus or minus the standard error. (B) D6P2T cells were left untreated or treated with rapamycin as in panel A. Cells were lysed, and the extracts were subjected to Western blotting for 4E-BP. α represents the position of the hypophosphorylated form of 4E-BP; β and γ represent positions of the hyperphosphorylated forms.
FIG. 6
Coexpression of 4E-BP does not affect p27 5′-UTR IRES activity. (A) D6P2T cells were cotransfected with the bicistronic construct carrying the full-length p27 5′-UTR and increasing amounts of the same vector encoding a mutant form of 4E-BP-1 which is constitutively active in inhibiting elF4E. The amount of DNA transfected was held constant by including the appropriate level of empty expression vector. Each extract was analyzed for luciferase and CAT activity. The luciferase/CAT ratio was determined by dividing the relative light units (luciferase) by the percentage of substrate converted to the acetylated form (CAT). All data represent the mean of a minimum of three replicates plus or minus the standard error. (B) D6P2T cells transfected as in panel A were harvested for Western blot analysis of 4E-BP levels. Equal amounts of protein from each extract were loaded in each lane. The position of the constitutively active 4E-BP mutant is indicated (5A-4EBP). The lower band, which is a nonspecific band recognized by the antibody, indicates that equal levels of protein were loaded in each lane. (C) NIH 3T3 cells were cotransfected with the bicistronic construct carrying the full-length p27 5′-UTR and 0, 1.5, or 3 μg of expression constructs encoding either the wild-type 4E-BP-1 (4EBP-WT) or constitutively active mutant 4E-BP (4EBP-5A mut). Extracts from transfected cells were assayed for CAT and luciferase activities. All data represent the mean of a minimum of three replicates plus or minus the standard error.
FIG. 7
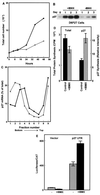
Enhanced p27 5′-UTR-mediated cap-independent translation in quiescent cells. (A) D6P2T cells were induced to differentiate by the addition of IBMX to 500 μM. At various times after the addition of IBMX, the cells were harvested by trypsinization and counted. The graph shows total cell number over time in untreated (solid line) and IBMX-treated (dashed line) cultures. (B) D6P2T cells were induced to differentiate by the addition of IBMX to 500 μM. Cell extracts were prepared over a 3-day period and used to detect p27 by Western blotting. (C) D6P2T cells were left untreated or induced to differentiate by addition of IBMX to 500 μM. After 3 days, the cells were harvested for polysome gradient analysis. Northern blots of RNA isolated from each gradient fraction were used to estimate the level of p27 mRNA. The percentage of the total p27 mRNA from gradient fractions from untreated cells (●) or IBMX-treated cells (▵) was plotted. The data are from a representative experiment. (D) D6P2T cells were pulse-labeled for 1 h with 35S-amino acids in the presence or absence of IBMX. Following the pulse, p27 was immunoprecipitated, and the resulting precipitate was run on a polyacrylamide gel. The gel was then subjected to autoradiography to determine the level of newly synthesized p27. The level of total protein synthesis was estimated by trichloroacetic acid precipitation of an aliquot of the extract representing 1.4 × 105 cells prior to immunoprecipitation. (E) D6P2T cells were transfected with pGL2CAT/Luc (vector) or pGL2CAT/Luc-p27UTR and then treated with or without IBMX. The normalized luciferase activity (luciferase/CAT ratio) for the transfected cells is shown. All data represent the mean of three replicates plus or minus the standard error.
FIG. 8
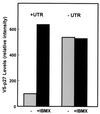
The p27 5′-UTR in its native context mediates inducible expression in D6P2T cells. Constructs encoding V5-epitope tagged p27 cDNA linked to either the first 3 nucleotides upstream of the AUG (−UTR) or the entire p27 5′-UTR (+UTR) were transfected into D6P2T cells. The cells were left untreated or treated with 500 μM IBMX. Two days after transfection, the cells were harvested and the level of V5-tagged p27 protein was determined by Western blotting with an anti-V5 antibody. The relative level of epitope-tagged p27 was determined by densitometric scanning. Data from a representative experiment of three repetitions are shown.
Similar articles
- Far upstream element binding protein 1 activates translation of p27Kip1 mRNA through its internal ribosomal entry site.
Zheng Y, Miskimins WK. Zheng Y, et al. Int J Biochem Cell Biol. 2011 Nov;43(11):1641-8. doi: 10.1016/j.biocel.2011.08.001. Epub 2011 Aug 9. Int J Biochem Cell Biol. 2011. PMID: 21855647 Free PMC article. - miR-181a regulates cap-dependent translation of p27(kip1) mRNA in myeloid cells.
Cuesta R, Martínez-Sánchez A, Gebauer F. Cuesta R, et al. Mol Cell Biol. 2009 May;29(10):2841-51. doi: 10.1128/MCB.01971-08. Epub 2009 Mar 9. Mol Cell Biol. 2009. PMID: 19273599 Free PMC article. - CUG-binding protein represses translation of p27Kip1 mRNA through its internal ribosomal entry site.
Zheng Y, Miskimins WK. Zheng Y, et al. RNA Biol. 2011 May-Jun;8(3):365-71. doi: 10.4161/rna.8.3.14804. Epub 2011 May 1. RNA Biol. 2011. PMID: 21508681 Free PMC article. - eIF4E activity is regulated at multiple levels.
Raught B, Gingras AC. Raught B, et al. Int J Biochem Cell Biol. 1999 Jan;31(1):43-57. doi: 10.1016/s1357-2725(98)00131-9. Int J Biochem Cell Biol. 1999. PMID: 10216943 Review. - Structure and functions of the translation initiation factor eIF4E and its role in cancer development and treatment.
Piserà A, Campo A, Campo S. Piserà A, et al. J Genet Genomics. 2018 Jan 20;45(1):13-24. doi: 10.1016/j.jgg.2018.01.003. Epub 2018 Feb 1. J Genet Genomics. 2018. PMID: 29396141 Review.
Cited by
- Mammalian target of rapamycin inhibitors induce tumor cell apoptosis in vivo primarily by inhibiting VEGF expression and angiogenesis.
Frost P, Berlanger E, Mysore V, Hoang B, Shi Y, Gera J, Lichtenstein A. Frost P, et al. J Oncol. 2013;2013:897025. doi: 10.1155/2013/897025. Epub 2013 Feb 28. J Oncol. 2013. PMID: 23533410 Free PMC article. - Upstream molecular signaling pathways of p27(Kip1) expression: effects of 4-hydroxytamoxifen, dexamethasone, and retinoic acids.
Eto I. Eto I. Cancer Cell Int. 2010 Feb 19;10:3. doi: 10.1186/1475-2867-10-3. Cancer Cell Int. 2010. PMID: 20170512 Free PMC article. - Regulation of expression by promoters versus internal ribosome entry site in the 5'-untranslated sequence of the human cyclin-dependent kinase inhibitor p27kip1.
Liu Z, Dong Z, Han B, Yang Y, Liu Y, Zhang JT. Liu Z, et al. Nucleic Acids Res. 2005 Jul 8;33(12):3763-71. doi: 10.1093/nar/gki680. Print 2005. Nucleic Acids Res. 2005. PMID: 16006622 Free PMC article. - Cold-inducible RNA-binding protein (CIRP) induces translation of the cell-cycle inhibitor p27Kip1.
Roilo M, Kullmann MK, Hengst L. Roilo M, et al. Nucleic Acids Res. 2018 Apr 6;46(6):3198-3210. doi: 10.1093/nar/gkx1317. Nucleic Acids Res. 2018. PMID: 29361038 Free PMC article. - Germline NPM1 mutations lead to altered rRNA 2'-O-methylation and cause dyskeratosis congenita.
Nachmani D, Bothmer AH, Grisendi S, Mele A, Bothmer D, Lee JD, Monteleone E, Cheng K, Zhang Y, Bester AC, Guzzetti A, Mitchell CA, Mendez LM, Pozdnyakova O, Sportoletti P, Martelli MP, Vulliamy TJ, Safra M, Schwartz S, Luzzatto L, Bluteau O, Soulier J, Darnell RB, Falini B, Dokal I, Ito K, Clohessy JG, Pandolfi PP. Nachmani D, et al. Nat Genet. 2019 Oct;51(10):1518-1529. doi: 10.1038/s41588-019-0502-z. Epub 2019 Sep 30. Nat Genet. 2019. PMID: 31570891 Free PMC article.
References
- Dahia P L, Aguiar R C, Honegger J, Fahlbush R, Jordan S, Lowe D G, Lu X, Clayton R N, Besser G M, Grossman A B. Mutation and expression analysis of the p27/kip1 gene in corticotrophin-secreting tumours. Oncogene. 1998;16:69–76. - PubMed
- De Benedetti A, Harris A L. eIF4E expression in tumors: its possible role in progression of malignancies. Int J Biochem Cell Biol. 1999;31:59–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous