Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway - PubMed (original) (raw)
Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway
M Furuhashi et al. Mol Cell Biol. 2001 Aug.
Abstract
Axin acts as a negative regulator in Wnt signaling through interaction with various molecules involved in this pathway, including beta-catenin, adenomatous polyposis coli, and glycogen synthase kinase 3beta. We show here that Axin also regulates the effects of Smad3 on the transforming growth factor beta (TGF-beta) signaling pathway. In the absence of activated TGF-beta receptors. Axin physically interacted with Smad3 through its C-terminal region located between the beta-catenin binding site and Dishevelled-homologous domain. An Axin homologue, Axil (also called conductin), also interacted with Smad3. In the absence of ligand stimulation, Axin was colocalized with Smad3 in the cytoplasm in vivo. Upon receptor activation, Smad3 was strongly phosphorylated by TGF-beta type I receptor (TbetaR-I) in the presence of Axin, and dissociated from TbetaR-I and Axin. Moreover, the transcriptional activity of TGF-beta was enhanced by Axin and repressed by an Axin mutant which is able to bind to Smad3. Axin may thus function as an adapter of Smad3, facilitating its activation by TGF-beta receptors for efficient TGF-beta signaling.
Figures
FIG. 1
Physical interaction between Axin and Smad3. (A) Interaction of Axin with Smad3 and a Smad3 mutant, Smad3D407E. COS7 cells were transfected with the indicated plasmids. Interaction between Smad3 and Axin in the presence and absence of a constitutively active TβR-I was determined by immunoprecipitation (IP) of Smad3 by anti-FLAG antibody followed by immunoblotting using anti-Myc antibody. The top panel shows the interaction between Smad3 and Axin, and the lower three panels show the expression of each protein. HA, hemagglutinin. (B) Direct interaction of Smad3 with Axin. GST-Smad3 or control GST was mixed with MBP-Axin or MBP. The samples were then incubated with glutathione-Sepharose 4B, and the precipitates were subjected to SDS-PAGE, followed by immunoblotting using anti-MBP antibody (lanes 1 to 3). As a control, MBP-Axin or MBP alone was directly subjected to SDS-PAGE (lanes 4 and 5). (C) Interaction of Smad1 through Smad5 with Axin. Experiments were performed as described for panel A but only in the absence of TβR-I(TD). The top panel shows the interaction between Smads and Axin. (D) Interaction between Axil and Smad3. Interaction of Smad3 with Axin or Axil was determined as described for panel C. (E) Interaction of Axin with Smad3 in HepG2 cells transfected with Axin alone. HepG2 cells were transfected with or without Myc-Axin and stimulated with TGF-β (100 pM) for the indicated periods. Interaction between endogenous Smad3 and Myc-Axin was determined by IP by anti-Smad3 antibody followed by immunoblotting using anti-Myc antibody. The top panel shows the interaction between Smad3 and Axin.
FIG. 2
Domains responsible for the interaction between Axin and Smad3. (A) Structure of rAxin and deletion mutants of rAxin used in the present study. The numbers indicate amino acid numbers (14). Dsh, Dishevelled family. (B) Physical interaction between Smad3 and various rAxin mutants. COS7 cells were transfected with the indicated plasmids. Interaction between Smad3 and rAxin mutants in the absence of TβR-I(TD) was determined by immunoprecipitation (IP) of Smad3 by anti-FLAG antibody followed by immunoblotting using anti-Myc antibody. The top panel shows the interaction between Smad3 and rAxin mutants, and the lower two panels show the expression of each protein. (C) Dvl-1 does not affect the interaction between Smad3 and Axin. COS7 cells were transfected with the indicated plasmids. Interaction between Smad3 and Axin in the presence of increasing amounts of Dvl-1 was examined by IP and immunoblotting as described for panel B. The top panel shows the interaction between Smad3 and Axin. Note that the level of expression of Axin decreased in the presence of large amounts of Dvl-1. HA, hemagglutinin. (D) Interaction of Axin with plasmids containing different portions of Smad3. Interaction was determined as described for panel B. Smad3 plasmids used in the present study are shown in the upper panel. FL, full-length; N, N-terminal MH1 domain; NL, N-terminal MH1 domain and linker region; LC, linker region and C-terminal MH2 domain; C, C-terminal MH2 domain.
FIG. 3
Recruitment of the Axin-Smad3 complex to TβR-I. (A) Interaction of Smad3D407E and Axin with TβR-I. COS7 cells were transfected with the indicated plasmids. Interaction of Smad3D407E or Axin with TβR-I was determined by immunoprecipitation (IP) of Smad3D407E or Axin by anti-FLAG antibody followed by immunoblotting of TβR-I using antihemagglutinin (anti-HA) antibody. The top panel shows the protein interaction, and the lower two panels show the expression of each protein. TβR-I plasmids used were as follows: wt, wild-type TβR-I; TD, TβR-I (TD); and KR, kinase-inactive form of TβR-I. (B) Interaction of Axin with TβR-I in the presence of increasing amounts of Samd3D407E. Interaction was determined as described for panel A. (C) Axin is not involved in the SARA-Smad3 complex. 293T cells were transfected with the indicated plasmids with increasing amounts of 6Myc-Axin. Interaction between SARA and Smad3 in the presence or absence of Axin was determined by IP of SARA by anti-FLAG antibody followed by immunoblotting of Smad3 and Axin using anti-Myc antibody. The top panel shows the interaction of SARA with Smad3 and Axin, and the lower panels show the expression of each protein.
FIG. 4
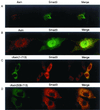
Subcellular localization of Smad3 and Axin. (A) Localization of Smad3 and Axin was examined by confocal microscopy. Anti-FLAG staining for FLAG-Smad3 (green) and anti-Axin antibody staining for Axin (red) followed by Alexa Fluor 488 and 568, respectively, were performed in transfected HepG2 cells. (B) Smad3 was dissociated from Axin upon receptor activation. Immunostaining was performed as described for panel A using transfected HepG2 cells in the presence of TβR-I(TD). (C and D) Localization of rAxin(1–713) (C) or rAxin(508–713) (D) and Smad3 was examined by anti-Myc staining for Axin and anti-Smad3 antibody staining for Smad3 as described for panel A.
FIG. 5
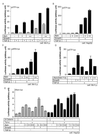
Phosphorylation of Smad3 in the presence of Axin. (A and B) Phosphorylation of endogenous Smad3 in Mv1Lu cells was determined in the presence and absence of Axin. Mv1Lu cells were infected with control adenovirus (Ad-LacZ) or Ad-Axin at a multiplicity of infection of 100 and treated with TGF-β (10 pM) for the indicated periods. Cell lysates were then immunoprecipitated (IP) by an anti-Smad2 and -Smad3 antibody, followed by immunoblotting using an anti-phospho-Smad3 antibody (P-Smad3). Expression of Axin and Smad2 and -3 is shown in the lower panels. In the panel demonstrating the expression of Smad2 and -3, the Smad3 bands were not well separated from the Smad2 bands. Intensities of the immunoblotted bands of phospho-Smad3 were therefore quantified compared to the Smad2 and -3 bands, and the values were plotted relative to the 0-min values. (C and D) Phosphorylation of transfected Smad3 by Axin. Mv1Lu cells were transiently transfected with the indicated plasmids and stimulated by TGF-β (10 pM) for the indicated periods. Phosphorylation of Smad3 was examined by FLAG IP followed by anti-phospho-Smad3 immunoblotting. Intensities of the immunoblotted bands of phospho-Smad3 were quantified compared to the Smad3 bands (FLAG IP followed by FLAG immunoblotting), and the values were plotted relative to the 0-min values.
FIG. 6
Enhancement of TGF-β activity by Axin. (A and B) The effects of Axin on the transcriptional activation of p3TP-lux were determined in the presence of the indicated plasmids with or without TGF-β stimulation (10 pM) in Mv1Lu cells (A) or HepG2 cells (B). For transfection of Axin, + and ++ represent 0.1 and 0.3 μg of rAxin DNA, respectively. Smad3 DNA was used at 0.3 μg. (C) The effect of Axin on pAR3-lux was examined with or without TGF-β (10 pM) in Mv1Lu cells. All cells were transfected with 0.4 μg of FoxH3 DNA. (D) Modulation of transcriptional activation of p3TP-lux by rAxin (508–713) was examined with or without TGF-β (10 pM) in Mv1Lu cells. For transfection of rAxin (508–713), + and ++ represent 0.3 and 1.0 μg of DNAs, respectively, transfected into cells. (E) Effects of Axin on Xtwn promoter activation in the presence of TGF-β and Wnt signaling. HepG2 cells were transfected with the indicated plasmids with or without TGF-β (10 pM). For transfection of the Axin DNA, +, ++, and +++ represent 0.1, 0.3, and 1.0 μg of DNA, respectively. Amounts of other DNAs were as follows: Smad3, 0.3 μg; Tcf-4, 0.01 μg; and β-catenin, 0.5 μg.
FIG. 7
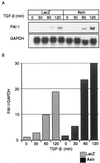
Enhancement of PAI-1 mRNA induction by TGF-β in the presence of Axin. Mv1Lu cells were infected with control adenovirus (Ad-LacZ) or Ad-Axin as described in the Fig. 5 legend and treated with TGF-β (10 pM) for the indicated periods. Northern blot analysis for PAI-1 was performed. Relative levels of PAI-1 expression were determined by densitometry and normalized to the levels of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as an internal control. The values were plotted relative to the 0-min values.
References
- Akiyama T. Wnt/β-catenin signaling. Cytokine Growth Factor Rev. 2000;11:273–282. - PubMed
- Behrens J, Jerchow B A, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science. 1998;280:596–599. - PubMed
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous