Identification of an overlapping binding domain on Cdc20 for Mad2 and anaphase-promoting complex: model for spindle checkpoint regulation - PubMed (original) (raw)
Identification of an overlapping binding domain on Cdc20 for Mad2 and anaphase-promoting complex: model for spindle checkpoint regulation
Y Zhang et al. Mol Cell Biol. 2001 Aug.
Abstract
Activation of the anaphase-promoting complex (APC) is required for anaphase initiation and for exit from mitosis in mammalian cells. Cdc20, which specifically recognizes APC substrates involved in the metaphase-to-anaphase transition, plays a pivotal role in APC activation through direct interaction with the APC. The activation of the APC by Cdc20 is prevented by the interaction of Cdc20 with Mad2 when the spindle checkpoint is activated. Using deletion mutagenesis and peptide mapping, we have identified the sequences in Cdc20 that target it to Mad2 and the APC, respectively. These sequences are distinct but overlapping, providing a possible structural explanation for the internal modulation of the APC-Cdc20 complex by Mad2. In the course of these studies, a truncation mutant of Cdc20 (1-153) that constitutively binds Mad2 but fails to bind the APC was identified. Overexpression of this mutant induces the formation of multinucleated cells and increases their susceptibility to undergoing apoptosis when treated with microtubule-inhibiting drugs. Our experiments demonstrate that disruption of the Mad2-Cdc20 interaction perturbs the mitotic checkpoint, leading to premature activation of the APC, sensitizing the cells to the cytotoxic effects of microtubule-inhibiting drugs.
Figures
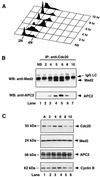
FIG. 1
Cell cycle-regulated association of Cdc20 with Mad2 and APC. A549 cells were synchronized at the G1/S boundary by a double-thymidine block, released into fresh medium, and collected every hour after release. (A) The profile of DNA content of released cells was analyzed by fluorescence-activated cell sorting. (B) Cells collected at indicated time points (hours) were lysed, cell extracts were immunoprecipitated with goat anti-Cdc20 polyclonal antibody, and the immunoprecipitates were analyzed by Western blotting with mouse anti-Mad2 monoclonal antibody (upper panel) and rabbit anti-APC2 polyclonal antibody (lower panel). Asynchronous cells (lane 1) were included as a control. (C) The levels of Cdc20, Mad2, APC2, and cyclin B proteins at indicated time points (hours) were determined by Western blot analysis. NS, nonsynchronous; IP, immunoprecipitation; WB, Western blotting; IgG, immunoglobulin G; LC, light chain; A, asynchronous.
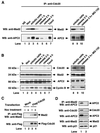
FIG. 2
Mitotic checkpoint-regulated association of Cdc20 with Mad2 and APC. (A) A549 cells were synchronized at prometaphase by nocodazole block. Mitotically arrested cells were collected by shake-off. Cells released into fresh medium were collected at indicated time points. To arrest cells in mitosis without activating the checkpoint, A549 cells were released from nocodazole into fresh medium containing 10 μM MG-132 (lane 10). Cell extracts (500 μg) were prepared at indicated time points and were immunoprecipitated with goat anti-Cdc20 polyclonal antibody, and the immunoprecipitates were analyzed by Western blotting with mouse anti-Mad2 monoclonal antibody (upper panel) and rabbit anti-APC2 polyclonal antibody (lower panel). Asynchronous cells (lane 1) and paclitaxel (lane 7) were included as controls. (B) The levels of Cdc20, Mad2, APC2, and cyclin B proteins at indicated time points and conditions were determined by Western blot analysis. (C) 293T cells were transfected with mock vector or Flag-tagged Cdc20 for 24 h. Transfected cells were treated with (+) or without (−) 0.4 μg of nocodazole/ml for 18 h. Cell extracts (1 mg) were immunoprecipitated with anti-Flag M2-conjugated agarose beads, and the immunoprecipitates were subjected to Western blot analysis with goat anti-Mad2 polyclonal antibody (upper panel). The level of Flag-tagged Cdc20 was determined by Western blotting with anti-Flag M5 monoclonal antibody (lower panel). (D) A549 cells were arrested in mitosis by nocodazole block, and cell extracts were immunoprecipitated with mouse anti-Mad2 monoclonal antibody either before (lane 1) or after (lane 2) immunodepletion with goat anti-Cdc20 polyclonal antibody. The immunoprecipitates were analyzed by Western blotting with rabbit anti-APC2 polyclonal antibody (upper panel). The amounts of Cdc20, APC2, Mad2, and p21 proteins in the supernatant before and after immunodepletion of Cdc20 were determined by Western blotting as indicated. IP, immunoprecipitation; NS, nonsynchronous; Noc, nocodazole; WB, Western blotting; A, asynchronous.
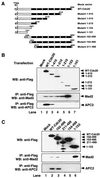
FIG. 3
The APC binding region of Cdc20 overlaps with but is distinct from the Mad2 binding region. (A) Schematic representation of Cdc20 deletion constructs. (B and C) 293T cells were transfected with equal amounts of indicated plasmids. After transfection for 24 h, cells were harvested, and expression of the corresponding WT and truncated mutant proteins was detected by Western blotting with anti-Flag M5 antibody (upper panels). Cell lysates were immunoprecipitated with anti-Flag M2 agarose beads. Precipitated proteins were separated by SDS-PAGE, transferred onto nylon membranes, and Western blotted with goat anti-Mad2 polyclonal antibody (middle panels) or rabbit anti-APC2 antibody (lower panels). WB, Western blotting; IP, immunoprecipitation.
FIG. 4
Peptide inhibition of Mad2 and APC binding to Ccd20. (A) Extracts from 293T cells transfected with Flag-tagged WT Cdc20 were incubated with indicated peptides (100 μM) in vitro at 4°C for 2 h. Flag-Cdc20 protein was immunoprecipitated using anti-Flag M2 agarose beads and then immunoblotted with goat anti-Mad2 polyclonal antibody (upper panel), rabbit anti-APC2 polyclonal antibody (middle panel), or anti-Flag M5 antibody (lower panel). (B) Extracts from 293T cells transfected with Flag-tagged WT Cdc20 were incubated with indicated concentrations of peptide 122–145 in vitro at 4°C for 2 h. Flag-Cdc20 protein was immunoprecipitated using anti-Flag M2 agarose beads and then immunoblotted with goat anti-Mad2 polyclonal antibody. (C) Extracts from 293T cells were incubated with biotin-conjugated peptide 122–145 or control biotin-conjugated peptide for 2 h at 4°C. Biotin-conjugated peptides were pulled down with streptavidin beads, and then biotin-peptide-associated Mad2 was detected by Western blotting with goat anti-Mad2 polyclonal antibody. (D) Comparison of the amino acid sequence 122 to 145 of hCdc20 with the aligned sequences from mouse, fission yeast, budding yeast, Drosophila melanogaster, and X. laevis. The amino acid mutations in yeast that inactivate the mitotic checkpoint are shown in red. The R132A mutation in humans also reduces the binding of Cdc20 to Mad2 (unpublished data). The alignment was generated with ClustalW using MacVector 6.5 software. Identical and conserved amino acids are shown as shaded areas. IP, immunoprecipitation; WB, Western blotting.
FIG. 5
Constitutive binding of 1–153 Cdc20 mutant to Mad2. 293T cells were transfected with equal amounts of indicated plasmids. After transfection for 24 h, cells were further treated with or without nocodazole for 18 h. Cells were harvested, and cell lysates were immunoprecipitated with anti-Flag agarose beads. Precipitated proteins were separated by SDS-PAGE, transferred onto nylon membranes, and Western blotted with goat anti-Mad2 polyclonal antibody (lower panel). The corresponding expression levels of WT and mutant proteins in transfected 293T cells were detected by Western blotting with anti-Flag M5 antibody (upper panel). Noc, nocodazole; IP, immunoprecipitation; WB, Western blotting.
FIG. 6
Phenotype of 293T cells overexpressing WT Cdc20 and mutants. (A) 293T cells were transfected with pFlag-CMV vectors encoding WT Cdc20, 1–153 Cdc20, 1–101 Cdc20, or 211–499 Cdc20. After transfection for 48 h, the transfected cells were detected by immunofluorescence using anti-Flag M5 antibody. DNA was stained with propidium iodide. (B) The above transfected 293T cells were further treated with nocodazole for 18 h. The mitotic index was analyzed by fluorescence microscopy using anti-Flag M5 antibody for positive cells and propidium iodide for chromosome DNA. The shaded bars and error bars represent the means and standard deviations, respectively, from at least two independent assessments of 100 cells each in a single experiment; similar results were obtained in two independent experiments. (C) 293T cells were transfected with pFlag-CMV vectors encoding 1–153 Cdc20, 1–101 Cdc20, or vector control for 48 h and were further treated with nocodazole for 18 h. The levels of the corresponding truncated mutant protein, endogenous Cdc20, and Mad2 were detected by Western blotting with anti-Flag M5 antibody, goat anti-Cdc20 polyclonal antibody, and mouse anti-Mad2 monoclonal antibody. Cell lysates were immunoprecipitated with goat anti-Cdc20 polyclonal antibody. Precipitated proteins were separated by SDS-PAGE, transferred onto nylon membranes, and Western blotted with mouse anti-Mad2 monoclonal antibody. PI, propidium iodide; Noc, nocodazole; WB, Western blotting; IP, immunoprecipitation.
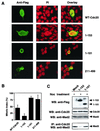
FIG. 7
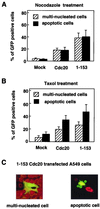
A549 cells were transfected with pEGFP vector encoding WT Cdc20 or 1–153 Cdc20 mutant for 24 h and were further treated with nocodazole (A) or paclitaxel (B) for 18 h. GFP fusion constructs were directly visualized by autofluorescence. Multinucleated cells with aberrantly shaped nuclei and apoptotic cells with nuclear condensation and fragmentation were determined by counting 100 GFP-positive cells visualized with propidium iodide staining. The data show the averages and standard deviations derived from three experiments. (C) Representative morphology of paclitaxel-treated A549 cells transfected with 1–153 Cdc20.
Similar articles
- Mad2 binding to Mad1 and Cdc20, rather than oligomerization, is required for the spindle checkpoint.
Sironi L, Melixetian M, Faretta M, Prosperini E, Helin K, Musacchio A. Sironi L, et al. EMBO J. 2001 Nov 15;20(22):6371-82. doi: 10.1093/emboj/20.22.6371. EMBO J. 2001. PMID: 11707408 Free PMC article. - Structure of the Mad2 spindle assembly checkpoint protein and its interaction with Cdc20.
Luo X, Fang G, Coldiron M, Lin Y, Yu H, Kirschner MW, Wagner G. Luo X, et al. Nat Struct Biol. 2000 Mar;7(3):224-9. doi: 10.1038/73338. Nat Struct Biol. 2000. PMID: 10700282 - Bub3 interaction with Mad2, Mad3 and Cdc20 is mediated by WD40 repeats and does not require intact kinetochores.
Fraschini R, Beretta A, Sironi L, Musacchio A, Lucchini G, Piatti S. Fraschini R, et al. EMBO J. 2001 Dec 3;20(23):6648-59. doi: 10.1093/emboj/20.23.6648. EMBO J. 2001. PMID: 11726501 Free PMC article. - Regulation of APC-Cdc20 by the spindle checkpoint.
Yu H. Yu H. Curr Opin Cell Biol. 2002 Dec;14(6):706-14. doi: 10.1016/s0955-0674(02)00382-4. Curr Opin Cell Biol. 2002. PMID: 12473343 Review. - The mitotic checkpoint: a signaling pathway that allows a single unattached kinetochore to inhibit mitotic exit.
Chan GK, Yen TJ. Chan GK, et al. Prog Cell Cycle Res. 2003;5:431-9. Prog Cell Cycle Res. 2003. PMID: 14593737 Review.
Cited by
- Spatiotemporal regulation of the anaphase-promoting complex in mitosis.
Sivakumar S, Gorbsky GJ. Sivakumar S, et al. Nat Rev Mol Cell Biol. 2015 Feb;16(2):82-94. doi: 10.1038/nrm3934. Nat Rev Mol Cell Biol. 2015. PMID: 25604195 Free PMC article. Review. - Rapid microtubule-independent dynamics of Cdc20 at kinetochores and centrosomes in mammalian cells.
Kallio MJ, Beardmore VA, Weinstein J, Gorbsky GJ. Kallio MJ, et al. J Cell Biol. 2002 Sep 2;158(5):841-7. doi: 10.1083/jcb.200201135. Epub 2002 Aug 26. J Cell Biol. 2002. PMID: 12196507 Free PMC article. - Changes in the localization of the Saccharomyces cerevisiae anaphase-promoting complex upon microtubule depolymerization and spindle checkpoint activation.
Melloy PG, Holloway SL. Melloy PG, et al. Genetics. 2004 Jul;167(3):1079-94. doi: 10.1534/genetics.103.025478. Genetics. 2004. PMID: 15280225 Free PMC article. - Spindle checkpoint requires Mad1-bound and Mad1-free Mad2.
Chung E, Chen RH. Chung E, et al. Mol Biol Cell. 2002 May;13(5):1501-11. doi: 10.1091/mbc.02-01-0003. Mol Biol Cell. 2002. PMID: 12006648 Free PMC article. - Closed MAD2 (C-MAD2) is selectively incorporated into the mitotic checkpoint complex (MCC).
Tipton AR, Tipton M, Yen T, Liu ST. Tipton AR, et al. Cell Cycle. 2011 Nov 1;10(21):3740-50. doi: 10.4161/cc.10.21.17919. Epub 2011 Nov 1. Cell Cycle. 2011. PMID: 22037211 Free PMC article.
References
- Amon A. The spindle checkpoint. Curr Opin Genet Dev. 1999;9:69–75. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases