Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-kappa B - PubMed (original) (raw)
Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-kappa B
R W Ganster et al. Proc Natl Acad Sci U S A. 2001.
Abstract
The human inducible nitric oxide synthase (hiNOS) gene is expressed in several disease states and is also important in the normal immune response. Previously, we described a cytokine-responsive enhancer between -5.2 and -6.1 kb in the 5'-flanking hiNOS promoter DNA, which contains multiple nuclear factor kappa beta (NF-kappa B) elements. Here, we describe the role of the IFN-Jak kinase-Stat (signal transducer and activator of transcription) 1 pathway for regulation of hiNOS gene transcription. In A549 human lung epithelial cells, a combination of cytokines tumor necrosis factor-alpha, interleukin-1 beta, and IFN-gamma (TNF-alpha, IL-1 beta, and IFN-gamma) function synergistically for induction of hiNOS transcription. Pharmacological inhibitors of Jak2 kinase inhibit cytokine-induced Stat 1 DNA-binding and hiNOS gene expression. Expression of a dominant-negative mutant Stat 1 inhibits cytokine-induced hiNOS reporter expression. Site-directed mutagenesis of a cis-acting DNA element at -5.8 kb in the hiNOS promoter identifies a bifunctional NF-kappa B/Stat 1 motif. In contrast, gel shift assays indicate that only Stat 1 binds to the DNA element at -5.2 kb in the hiNOS promoter. Interestingly, Stat 1 is repressive to basal and stimulated iNOS mRNA expression in 2fTGH human fibroblasts, which are refractory to iNOS induction. Overexpression of NF-kappa B activates hiNOS promoter-reporter expression in Stat 1 mutant fibroblasts, but not in the wild type, suggesting that Stat 1 inhibits NF-kappa B function in these cells. These results indicate that both Stat 1 and NF-kappa B are important in the regulation of hiNOS transcription by cytokines in a complex and cell type-specific manner.
Figures
Figure 1
Overlapping NF-κB and Stat 1 elements at −5.2 kb and −5.8 kb in the hiNOS promoter.
Figure 2
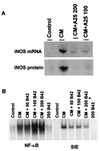
The effect of Jak kinase inhibitors on hiNOS expression and on cytokine induction of nuclear NF-κB or Stat 1 DNA-binding. (A) Northern and Western blot analysis of hiNOS mRNA and protein induced by CM. The Jak kinase inhibitor (tyrophostin A25) inhibits cytokine-induced iNOS mRNA and protein expression in a dose-dependent fashion. (B) The gel shift experiment shows the effects of the Jak 2 kinase inhibitor tyrophostin B42 on the nuclear DNA-binding activities of both NF-κB and Stat 1. Nuclear protein extracts were prepared from cells exposed for 2 h to a cytokine mixture in the absence or presence of tyrophostin B42 as indicated. The nuclear proteins were subjected to EMSA, using the consensus NF-κB or hSIE oligonucleotide probes. Blots shown are representative of three similar experiments.
Figure 3
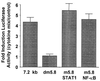
The cis-acting transcription element at −5.8 kb in the hiNOS promoter is a bifunctional, composite NF-κB/GAS element. The graph depicts the cytokine-induced expression of the −7.2-kb hiNOS promoter–luciferase reporter and various mutant derivatives shown in Fig. 1. Mutation of both the NF-κB and the Stat 1 elements eliminate cytokine induction of the hiNOS reporter plasmid, whereas mutation of the NF-κB or Stat 1 sequence individually had no effect on hiNOS promoter activity (n = 4–6 transfections per condition).
Figure 4
Inflammatory cytokines induce distinct NF-κB or Stat 1–DNA complexes at the −5.8 kb hiNOS promoter element. The figure is a gel shift assay analyzing the induction of nuclear DNA-binding proteins in response to either TNF-α, IFN-γ, or a combination in nuclear extracts from human A549 lung epithelium. Antibody supershift assays indicate that TNF-α induces a protein–DNA complex containing NF-κB protein, whereas IFN-γ induces a Stat 1–DNA complex. Blots shown are representative of two similar experiments.
Figure 5
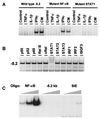
The cytokine responsive element at −5.2 kb in the hiNOS promoter is a functional Stat 1 DNA-binding sequence. (A) Only IFN-γ alone or as part of CM induces a protein/DNA-binding complex with the −5.2-kb element. Mutation of the NF-κB domain does not alter binding, but mutation of the Stat 1 site abolishes all binding. (B) Antibody supershift shows that the protein–DNA complex at −5.2 kb is recognized exclusively by Stat 1 antibody. (C) Purified recombinant NF-κB protein binds to a consensus NF-κB oligo, but not to the −5.2 kb or to a consensus Stat 1 (hSIE) element. Blots shown are representative of three similar experiments.
Figure 6
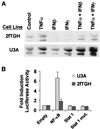
Interferons and Stat 1 repress hiNOS mRNA and NF-κB-mediated hiNOS transcription in a human fibroblast cell line. (A) The Northern blot shows hiNOS mRNA expression in response to various combinations of cytokines as labeled. IFN-β or IFN-γ inhibit basal and stimulated hiNOS mRNA expression in the wild-type 2fTGH human fibroblasts, but not in the Stat 1-null U3A cells. (B) Analysis of hiNOS promoter–luciferase reporter plasmids in human 2fTGH and U3A fibroblasts indicate that Stat 1 can function as a repressor of NF-κB-induced iNOS transcription. The figure illustrates the fold induction of a hiNOS–luciferase reporter in cells that were cotransfected with empty vector or vectors that express either p50 + p65 NF-κB protein, wild-type Stat 1, or dominant-negative mutant Stat 1 protein. Notice that NF-κB overexpression will induce a significant hiNOS expression only in the Stat 1 mutant U3A cells. Blots shown are representative of three similar experiments.
Similar articles
- Identification of a negative response element in the human inducible nitric-oxide synthase (hiNOS) promoter: The role of NF-kappa B-repressing factor (NRF) in basal repression of the hiNOS gene.
Feng X, Guo Z, Nourbakhsh M, Hauser H, Ganster R, Shao L, Geller DA. Feng X, et al. Proc Natl Acad Sci U S A. 2002 Oct 29;99(22):14212-7. doi: 10.1073/pnas.212306199. Epub 2002 Oct 15. Proc Natl Acad Sci U S A. 2002. PMID: 12381793 Free PMC article. - Up-Regulation of Human Inducible Nitric Oxide Synthase by p300 Transcriptional Complex.
Guo Z, Zheng L, Liao X, Geller D. Guo Z, et al. PLoS One. 2016 Jan 11;11(1):e0146640. doi: 10.1371/journal.pone.0146640. eCollection 2016. PLoS One. 2016. PMID: 26751080 Free PMC article. - Cytokine induction of NO synthase II in human DLD-1 cells: roles of the JAK-STAT, AP-1 and NF-kappaB-signaling pathways.
Kleinert H, Wallerath T, Fritz G, Ihrig-Biedert I, Rodriguez-Pascual F, Geller DA, Förstermann U. Kleinert H, et al. Br J Pharmacol. 1998 Sep;125(1):193-201. doi: 10.1038/sj.bjp.0702039. Br J Pharmacol. 1998. PMID: 9776360 Free PMC article. - The harmony of the spheres: inducible nitric oxide synthase and related genes in pancreatic beta cells.
Eizirik DL, Flodström M, Karlsen AE, Welsh N. Eizirik DL, et al. Diabetologia. 1996 Aug;39(8):875-90. doi: 10.1007/BF00403906. Diabetologia. 1996. PMID: 8858209 Review. - Molecular regulation of the human inducible nitric oxide synthase (iNOS) gene.
Taylor BS, Geller DA. Taylor BS, et al. Shock. 2000 Jun;13(6):413-24. doi: 10.1097/00024382-200006000-00001. Shock. 2000. PMID: 10847627 Review.
Cited by
- Nuclear factor-kappaB motif and interferon-alpha-stimulated response element co-operate in the activation of guanylate-binding protein-1 expression by inflammatory cytokines in endothelial cells.
Naschberger E, Werner T, Vicente AB, Guenzi E, Töpolt K, Leubert R, Lubeseder-Martellato C, Nelson PJ, Stürzl M. Naschberger E, et al. Biochem J. 2004 Apr 15;379(Pt 2):409-20. doi: 10.1042/BJ20031873. Biochem J. 2004. PMID: 14741045 Free PMC article. - Transcriptional suppression of cytokine-induced iNOS gene expression by IL-13 through IRF-1/ISRE signaling.
Shao L, Guo Z, Geller DA. Shao L, et al. Biochem Biophys Res Commun. 2007 Oct 26;362(3):582-6. doi: 10.1016/j.bbrc.2007.07.203. Epub 2007 Aug 21. Biochem Biophys Res Commun. 2007. PMID: 17723228 Free PMC article. - Daphnetin reduces endotoxin lethality in mice and decreases LPS-induced inflammation in Raw264.7 cells via suppressing JAK/STATs activation and ROS production.
Shen L, Zhou T, Wang J, Sang X, Lan L, Luo L, Yin Z. Shen L, et al. Inflamm Res. 2017 Jul;66(7):579-589. doi: 10.1007/s00011-017-1039-1. Epub 2017 Apr 13. Inflamm Res. 2017. PMID: 28409189 - Oct-1 cooperates with the TATA binding initiation complex to control rapid transcription of human iNOS.
Reveneau S, Petrakis TG, Goldring CE, Chantôme A, Jeannin JF, Pance A. Reveneau S, et al. Cell Mol Life Sci. 2012 Aug;69(15):2609-19. doi: 10.1007/s00018-012-0939-z. Epub 2012 Feb 19. Cell Mol Life Sci. 2012. PMID: 22349263 Free PMC article. - The homeoprotein DLX4 controls inducible nitric oxide synthase-mediated angiogenesis in ovarian cancer.
Trinh B, Ko SY, Haria D, Barengo N, Naora H. Trinh B, et al. Mol Cancer. 2015 Apr 30;14:97. doi: 10.1186/s12943-015-0368-3. Mol Cancer. 2015. PMID: 25924901 Free PMC article.
References
- Taylor B S, de Vera M E, Ganster R W, Wang Q, Shapiro R A, Morris S M, Jr, Billiar T R, Geller D A. J Biol Chem. 1998;273:15148–15156. - PubMed
- Ganster R W, Geller D A. In: Nitric Oxide: Biology and Pathobiology. Ignarro L, editor. San Diego: Academic; 2000. pp. 129–156.
- Xie Q W, Cho H J, Calaycay J, Mumford R A, Swiderek K M, Lee T D, Ding A, Troso T, Nathan C. Science. 1992;256:225–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 GM019877/GM/NIGMS NIH HHS/United States
- R01 GM052021/GM/NIGMS NIH HHS/United States
- F32 GM-19877/GM/NIGMS NIH HHS/United States
- R01-GM-52021/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous