Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice - PubMed (original) (raw)
Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice
G Nicolas et al. Proc Natl Acad Sci U S A. 2001.
Abstract
We previously reported the disruption of the murine gene encoding the transcription factor USF2 and its consequences on glucose-dependent gene regulation in the liver. We report here a peculiar phenotype of Usf2(-/-) mice that progressively develop multivisceral iron overload; plasma iron overcomes transferrin binding capacity, and nontransferrin-bound iron accumulates in various tissues including pancreas and heart. In contrast, the splenic iron content is strikingly lower in knockout animals than in controls. To identify genes that may account for the abnormalities of iron homeostasis in Usf2(-/-) mice, we used suppressive subtractive hybridization between livers from Usf2(-/-) and wild-type mice. We isolated a cDNA encoding a peptide, hepcidin (also referred to as LEAP-1, for liver-expressed antimicrobial peptide), that was very recently purified from human blood ultrafiltrate and from urine as a disulfide-bonded peptide exhibiting antimicrobial activity. Accumulation of iron in the liver has been recently reported to up-regulate hepcidin expression, whereas our data clearly show that a complete defect in hepcidin expression is responsible for progressive tissue iron overload. The striking similarity of the alterations in iron metabolism between HFE knockout mice, a murine model of hereditary hemochromatosis, and the Usf2(-/-) hepcidin-deficient mice suggests that hepcidin may function in the same regulatory pathway as HFE. We propose that hepcidin acts as a signaling molecule that is required in conjunction with HFE to regulate both intestinal iron absorption and iron storage in macrophages.
Figures
Figure 1
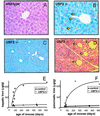
Iron accumulation in liver and pancreas of _Usf2_−/− mice. Liver and pancreas were fixed in formaldehyde and stained with the Perls' stain for iron. Nonheme iron stains blue. Liver sections are from an 8-month-old wild-type mice (×50) (A), an 8-month-old _Usf2_−/− littermate (B), and a 19-month-old _Usf2_−/− mouse (×10) (C). Pancreas section in D is from an 8-month-old _Usf2_−/− mouse (×12.5). Arrowheads in C indicate iron in the nucleus of the hepatocyte. Arrowheads in D point to islets of Langerhans scattered throughout the exocrine tissue. (E and F) Age-dependent hepatic and pancreatic nonheme iron concentration (micrograms of iron per gram dry tissue) as measured in control (wild-type and heterozygote mice, ▴) and _Usf2_−/− mice (□).
Figure 2
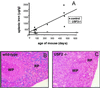
Iron content in spleen of _Usf2_−/− mice. (A) Age-dependent splenic nonheme iron concentration (micrograms of iron per gram dry tissue) as measured in control (wild-type and heterozygote mice, ▴) and _Usf2_−/− mice (□). Spleen section from a representative 8-month-old wild-type mouse (×20) (B) and an 8-month-old _Usf2_−/− littermate (×20) (C) stained with the Perls' stain for iron. RP, red pulp; WP, white pulp.
Figure 3
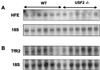
HFE and TFR2 mRNA content in liver of wild-type and _Usf2_−/− animals as determined by Northern blot analysis. Twenty micrograms of total liver RNAs from wild-type mice and _Usf2_−/− mice (from 3 to 11 months old) were electrophoresed and blotted. Blots were hybridized with a 32P-labeled probe (made by PCR, as described in Materials and Methods) for HFE (A) and RTf2 (B).
Figure 4
Genomic organization of Usf2 and hepcidin genes. Schematic representation (not to scale) of the locus region encompassing the Usf2 and hepcidin genes. The targeted allele is represented with the betageo cassette insertion in exon 7 (11). Data are the result of genomic RP23–22G9 clone (GenBank). So far, no data are available concerning the orientation and the distance between the two hepcidin genes. The Southern blot in the right of the figure is from tail DNA of wild-type, heterozygote, and homozygote mice digested by _Bgl_II and hybridized with the HEPC1 probe. Two bands of the expected size, 12.4 and 5.1 kbp, were detected, whatever the genotype. The same bands were revealed by using the Usf2 probe.
Figure 5
Hepcidin mRNA content in liver of wild-type, Usf2+/−, and _Usf2_−/− animals as determined by Northern blot analysis and RT-PCR. (A) Twenty micrograms of total liver RNAs from wild-type, Usf2+/−, and _Usf2_−/− animals (between 3 and 11 months old) were electrophoresed and blotted. The blot was hybridized with a 32P-labeled HEPC probe (as described in Materials and Methods), which most likely recognized both _HEPC_1 and _HEPC_2 transcripts. (B) Specific _HEPC_1 and _HEPC_2 levels were measured by RT-PCR as described in Materials and Methods. Following PCR, the amplified products (171 bp for _HEPC_1 or _HEPC_2 and 250 bp for β-actin) were separated by electrophoresis on 1.5% agarose gel. Neither _HEPC_1 nor _HEPC_2 specific primers were able to reamplify HEPC2 and HEPC1 PCR products, respectively, demonstrating the high specificity of each pair of primers (not shown).
Figure 6
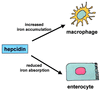
Hypothetical model for hepcidin as a key regulator of iron homeostasis. In this model, hepcidin prevents iron overload by reducing iron transport in the enterocyte and by programming macrophages to retain iron. In _Usf2_−/− mice, the hepcidin defect would be responsible for increased intestinal iron transport and reduced macrophage iron stores.
Comment in
- Hepcidin: a putative iron-regulatory hormone relevant to hereditary hemochromatosis and the anemia of chronic disease.
Fleming RE, Sly WS. Fleming RE, et al. Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8160-2. doi: 10.1073/pnas.161296298. Proc Natl Acad Sci U S A. 2001. PMID: 11459944 Free PMC article. Review. No abstract available.
Similar articles
- Hepcidin, a candidate modifier of the hemochromatosis phenotype in mice.
Nicolas G, Andrews NC, Kahn A, Vaulont S. Nicolas G, et al. Blood. 2004 Apr 1;103(7):2841-3. doi: 10.1182/blood-2003-09-3358. Epub 2003 Dec 4. Blood. 2004. PMID: 14656876 - Hepcidin: a putative iron-regulatory hormone relevant to hereditary hemochromatosis and the anemia of chronic disease.
Fleming RE, Sly WS. Fleming RE, et al. Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8160-2. doi: 10.1073/pnas.161296298. Proc Natl Acad Sci U S A. 2001. PMID: 11459944 Free PMC article. Review. No abstract available. - Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis.
Lesbordes-Brion JC, Viatte L, Bennoun M, Lou DQ, Ramey G, Houbron C, Hamard G, Kahn A, Vaulont S. Lesbordes-Brion JC, et al. Blood. 2006 Aug 15;108(4):1402-5. doi: 10.1182/blood-2006-02-003376. Epub 2006 Mar 30. Blood. 2006. PMID: 16574947 - Hemochromatosis and pregnancy: iron stores in the Hfe-/- mouse are not reduced by multiple pregnancies.
Neves JV, Olsson IA, Porto G, Rodrigues PN. Neves JV, et al. Am J Physiol Gastrointest Liver Physiol. 2010 Apr;298(4):G525-9. doi: 10.1152/ajpgi.00449.2009. Epub 2010 Jan 28. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20110460 - [Pathophysiology and genetics of classic HFE (type 1) hemochromatosis].
Loréal O, Ropert M, Mosser A, Déhais V, Deugnier Y, David V, Brissot P, Jouanolle AM. Loréal O, et al. Presse Med. 2007 Sep;36(9 Pt 2):1271-7. doi: 10.1016/j.lpm.2007.03.038. Epub 2007 May 22. Presse Med. 2007. PMID: 17521857 Review. French.
Cited by
- Validity of serum and urinary hepcidin as biomarkers for late-onset sepsis in premature infants.
Sherbiny HS, Mostafa HAF, Sherief LM, Kamal NM, El-Shal AS, Abdel-El Halm MM, Khan HY, Ali ASA. Sherbiny HS, et al. Ther Adv Chronic Dis. 2022 Sep 6;13:20406223221122527. doi: 10.1177/20406223221122527. eCollection 2022. Ther Adv Chronic Dis. 2022. PMID: 36093263 Free PMC article. - Hemochromatosis: a model of metal-related human toxicosis.
Brissot P, Cavey T, Ropert M, Gaboriau F, Loréal O. Brissot P, et al. Environ Sci Pollut Res Int. 2018 Jan;25(3):2007-2013. doi: 10.1007/s11356-016-7576-2. Epub 2016 Sep 15. Environ Sci Pollut Res Int. 2018. PMID: 27628916 - Supplementation with >Your< Iron Syrup Corrects Iron Status in a Mouse Model of Diet-Induced Iron Deficiency.
Pirman T, Lenardič A, Nemec Svete A, Horvat S. Pirman T, et al. Biology (Basel). 2021 Apr 22;10(5):357. doi: 10.3390/biology10050357. Biology (Basel). 2021. PMID: 33922324 Free PMC article. - Anemia in the elderly.
Berliner N. Berliner N. Trans Am Clin Climatol Assoc. 2013;124:230-7. Trans Am Clin Climatol Assoc. 2013. PMID: 23874029 Free PMC article. - HFE inhibits type I IFNs signaling by targeting the SQSTM1-mediated MAVS autophagic degradation.
Liu J, Wu X, Wang H, Wei J, Wu Q, Wang X, Yan Y, Cui J, Min J, Wang F, Zhou J. Liu J, et al. Autophagy. 2021 Aug;17(8):1962-1977. doi: 10.1080/15548627.2020.1804683. Epub 2020 Aug 18. Autophagy. 2021. PMID: 32746697 Free PMC article.
References
- Andrews N C. Nat Rev Genet. 2000;1:208–217. - PubMed
- Fleming M D, Trenor C C, Su M A, Foernzler D, Beier D R, Dietrich W F, Andrews N C. Nat Genet. 1997;16:383–386. - PubMed
- Gunshin H, Mackenzie B, Berger U V, Gunshin Y, Romero M F, Boron W F, Nussberger S, Gollan J L, Hediger M A. Nature (London) 1997;388:482–488. - PubMed
- Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt S J, Moynihan J, Paw B H, Drejer A, Barut B, Zapata A, et al. Nature (London) 2000;403:776–781. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases