The LD4 motif of paxillin regulates cell spreading and motility through an interaction with paxillin kinase linker (PKL) - PubMed (original) (raw)
The LD4 motif of paxillin regulates cell spreading and motility through an interaction with paxillin kinase linker (PKL)
K A West et al. J Cell Biol. 2001.
Abstract
The small GTPases of the Rho family are intimately involved in integrin-mediated changes in the actin cytoskeleton that accompany cell spreading and motility. The exact means by which the Rho family members elicit these changes is unclear. Here, we demonstrate that the interaction of paxillin via its LD4 motif with the putative ARF-GAP paxillin kinase linker (PKL) (Turner et al., 1999), is critically involved in the regulation of Rac-dependent changes in the actin cytoskeleton that accompany cell spreading and motility. Overexpression of a paxillin LD4 deletion mutant (paxillinDeltaLD4) in CHO.K1 fibroblasts caused the generation of multiple broad lamellipodia. These morphological changes were accompanied by an increase in cell protrusiveness and random motility, which correlated with prolonged activation of Rac. In contrast, directional motility was inhibited. These alterations in morphology and motility were dependent on a paxillin-PKL interaction. In cells overexpressing paxillinDeltaLD4 mutants, PKL localization to focal contacts was disrupted, whereas that of focal adhesion kinase (FAK) and vinculin was not. In addition, FAK activity during spreading was not compromised by deletion of the paxillin LD4 motif. Furthermore, overexpression of PKL mutants lacking the paxillin-binding site (PKLDeltaPBS2) induced phenotypic changes reminiscent of paxillinDeltaLD4 mutant cells. These data suggest that the paxillin association with PKL is essential for normal integrin-mediated cell spreading, and locomotion and that this interaction is necessary for the regulation of Rac activity during these events.
Figures
Figure 1.
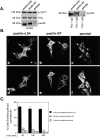
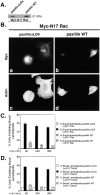
Expression of paxillin LD4 deletion generates multiple broad lamellipodia during cell spreading. (A) Immunoprecipitation using avian-specific paxillin antisera (Pax1, right) and Western immunoblotting of total cell lysates (left) confirm the relative levels of paxillin, PKL, and p130cas protein, and demonstrates the overexpression of avian paxillin in the paxillinΔLD4 and paxillin WT cells compared with parental nontransfected CHO.K1 cells. (B, a and d) CHO.K1 cells ectopically expressing avian paxillin with the deletion of LD4 (paxillinΔLD4); (b and e) CHO.K1 cells ectopically expressing full-length wild-type avian paxillin (paxillin WT); (c and f) parental nontransfected CHO.K1 cells. CHO.K1 cells were respread on fibronectin-coated (Fn) coverslips (10 μg/ml) for 60, 240, and 360 min, and ectopic paxillin (Pax1, a and b), endogenous paxillin (c), and actin (d–f) were examined. PaxillinΔLD4 cells exhibit a dramatic increase in the generation of broad lamellipodia with ectopic paxillin localizing to the cell periphery in focal contacts (a, arrows). Tail-like retraction fibers were frequently observed (a, arrowhead). Double arrows in a show ectopic paxillin in central focal contacts. Arrows in b and c show ectopic and endogenous paxillin in focal contacts, respectively. Images of the cells were captured at 240 min and are representative of the differences in cell morphology observed at the time points tested; i.e., 60, 240, and 360 min. (C) The number of cells exhibiting multiple broad lamellipodia (as exemplified by the cell pictured in B, a and d) was quantified by counting >200 cells per time point and indicates the dramatic increase in these structures in paxillinΔLD4 cells. Values are the average of experiments performed in triplicate.
Figure 2.
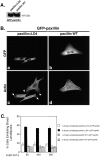
Reintroduction of wild-type paxillin into paxillinΔLD4 cells rescues the normal spreading phenotype. (A) Western blot analysis using GFP polyclonal antisera was used to confirm the presence of the ectopic GFP–paxillin protein. (B) PaxillinΔLD4 and paxillin wild-type cells were transiently transfected with GFP-paxillinΔLD4 and subjected to spreading assays on fibronectin for 60, 240, and 360 min, and GFP–paxillin transfectants were visualized by GFP fluorescence (a and b), and actin, by RITC-phalloidin (c and d). Arrows in c indicate paxillinΔLD4 cells expressing GFP–paxillin lacking broad lamellipodia, whereas arrowheads in c demonstrate the presence of these structuresin a cell lacking GFP-paxillin. Images of the cells were captured at the 240-min time point and are representative of the differences in cell morphology observed at all time points. (C) The number of cells exhibiting multiple broad lamellipodia was quantified by counting >200 cells per time point. Values are the average of experiments performed in triplicate.
Figure 3.
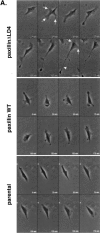
Overexpression of paxillinΔLD4 affects the protrusive activity and motility of cells. (A) PaxillinΔLD4, paxillin WT, and parental nontransfected CHO.K1 cells were plated on fibronectin (2 μg/ml), and motility was analyzed by time-lapse video microscopy. PaxillinΔLD4 cells were much more dynamic than paxillin WT and parental nontransfected cells, extending multiple lamellipodia (arrows). Long retraction fiber-like extensions were also observed (arrowhead). (B) Quantification of protrusiveness demonstrates an increase in cell area (in μm2) of paxillinΔLD4 cells, as compared with paxillin WT and parental nontransfected cells. (C) Quantification of random motility on fibronectin (2 μg/ml) demonstrates an increase in locomotion (in μm/hr) in paxillinΔLD4 cells, as compared with paxillin WT and parental nontransfected cells. (D) Quantification of random motility to fibronectin (10 μg/ml) reveals a striking increase in the percentage of paxillinΔLD4 cells migrating to immobilized fibronectin, as compared with paxillin WT, paxillinΔLD2, and parental nontransfected cells. Values are the average of modified Boyden chamber assays performed in quadruplicate. The migration of parental nontransfected CHO.K1 cells was set to 100%, and the other cell types were measured against this value. (E) Scrape wound assays using paxillinΔLD4 and paxillin WT cells plated in 35-mm tissue culture dishes in complete media at a confluent density and scored with a micropipet tip demonstrate that, although paxillin WT cells are able close the wound, paxillinΔLD4 are severely retarded in this capacity. The images are representative of experiments performed in triplicate.
Figure 3.
Overexpression of paxillinΔLD4 affects the protrusive activity and motility of cells. (A) PaxillinΔLD4, paxillin WT, and parental nontransfected CHO.K1 cells were plated on fibronectin (2 μg/ml), and motility was analyzed by time-lapse video microscopy. PaxillinΔLD4 cells were much more dynamic than paxillin WT and parental nontransfected cells, extending multiple lamellipodia (arrows). Long retraction fiber-like extensions were also observed (arrowhead). (B) Quantification of protrusiveness demonstrates an increase in cell area (in μm2) of paxillinΔLD4 cells, as compared with paxillin WT and parental nontransfected cells. (C) Quantification of random motility on fibronectin (2 μg/ml) demonstrates an increase in locomotion (in μm/hr) in paxillinΔLD4 cells, as compared with paxillin WT and parental nontransfected cells. (D) Quantification of random motility to fibronectin (10 μg/ml) reveals a striking increase in the percentage of paxillinΔLD4 cells migrating to immobilized fibronectin, as compared with paxillin WT, paxillinΔLD2, and parental nontransfected cells. Values are the average of modified Boyden chamber assays performed in quadruplicate. The migration of parental nontransfected CHO.K1 cells was set to 100%, and the other cell types were measured against this value. (E) Scrape wound assays using paxillinΔLD4 and paxillin WT cells plated in 35-mm tissue culture dishes in complete media at a confluent density and scored with a micropipet tip demonstrate that, although paxillin WT cells are able close the wound, paxillinΔLD4 are severely retarded in this capacity. The images are representative of experiments performed in triplicate.
Figure 3.
Overexpression of paxillinΔLD4 affects the protrusive activity and motility of cells. (A) PaxillinΔLD4, paxillin WT, and parental nontransfected CHO.K1 cells were plated on fibronectin (2 μg/ml), and motility was analyzed by time-lapse video microscopy. PaxillinΔLD4 cells were much more dynamic than paxillin WT and parental nontransfected cells, extending multiple lamellipodia (arrows). Long retraction fiber-like extensions were also observed (arrowhead). (B) Quantification of protrusiveness demonstrates an increase in cell area (in μm2) of paxillinΔLD4 cells, as compared with paxillin WT and parental nontransfected cells. (C) Quantification of random motility on fibronectin (2 μg/ml) demonstrates an increase in locomotion (in μm/hr) in paxillinΔLD4 cells, as compared with paxillin WT and parental nontransfected cells. (D) Quantification of random motility to fibronectin (10 μg/ml) reveals a striking increase in the percentage of paxillinΔLD4 cells migrating to immobilized fibronectin, as compared with paxillin WT, paxillinΔLD2, and parental nontransfected cells. Values are the average of modified Boyden chamber assays performed in quadruplicate. The migration of parental nontransfected CHO.K1 cells was set to 100%, and the other cell types were measured against this value. (E) Scrape wound assays using paxillinΔLD4 and paxillin WT cells plated in 35-mm tissue culture dishes in complete media at a confluent density and scored with a micropipet tip demonstrate that, although paxillin WT cells are able close the wound, paxillinΔLD4 are severely retarded in this capacity. The images are representative of experiments performed in triplicate.
Figure 3.
Overexpression of paxillinΔLD4 affects the protrusive activity and motility of cells. (A) PaxillinΔLD4, paxillin WT, and parental nontransfected CHO.K1 cells were plated on fibronectin (2 μg/ml), and motility was analyzed by time-lapse video microscopy. PaxillinΔLD4 cells were much more dynamic than paxillin WT and parental nontransfected cells, extending multiple lamellipodia (arrows). Long retraction fiber-like extensions were also observed (arrowhead). (B) Quantification of protrusiveness demonstrates an increase in cell area (in μm2) of paxillinΔLD4 cells, as compared with paxillin WT and parental nontransfected cells. (C) Quantification of random motility on fibronectin (2 μg/ml) demonstrates an increase in locomotion (in μm/hr) in paxillinΔLD4 cells, as compared with paxillin WT and parental nontransfected cells. (D) Quantification of random motility to fibronectin (10 μg/ml) reveals a striking increase in the percentage of paxillinΔLD4 cells migrating to immobilized fibronectin, as compared with paxillin WT, paxillinΔLD2, and parental nontransfected cells. Values are the average of modified Boyden chamber assays performed in quadruplicate. The migration of parental nontransfected CHO.K1 cells was set to 100%, and the other cell types were measured against this value. (E) Scrape wound assays using paxillinΔLD4 and paxillin WT cells plated in 35-mm tissue culture dishes in complete media at a confluent density and scored with a micropipet tip demonstrate that, although paxillin WT cells are able close the wound, paxillinΔLD4 are severely retarded in this capacity. The images are representative of experiments performed in triplicate.
Figure 4.
Rac activity is prolonged in paxillinΔLD4 cells. (A) PaxillinΔLD4 and paxillin WT cells were detached and allowed to spread on fibronectin for 0, 5, 15, 60, 240, and 360 min. At each time point, cell lysates were prepared, and then activated Rac was precipitated using GST fusion proteins of the p21-binding domain of PAK (GST–PBD). Total and active Rac was assessed by immunoblot analysis with anti-Rac monoclonal antibodies and revealed a gradual prolonged Rac activation in paxillinΔLD4 cells compared with paxillin WT cells, which exhibit an initial rise in activity before returning to baseline activity. (B) The activation of Rac in paxillinΔLD4 and paxillin WT cells during spreading was quantified by measuring the ratio of GST–PBD-bound Rac to total Rac. The t = 0 value of paxillin WT cells was set to 1, and all other values were measured against it. Values are the average of experiments performed in triplicate. The inset depicts Rac activation in paxillinΔLD4 and paxillin WT cells between 0 and 70 min.
Figure 5.
Introduction of dominant-negative forms of Rac and Cdc42 abrogate the morphological changes observed in paxillinΔLD4 cells. (A) Western blot analysis using monoclonal anti-Myc antibodies was used to confirm the presence of the ectopic Myc-tagged N17 Rac in paxilinΔLD4 and wild-type paxillin-transfected cells. (B) PaxillinΔLD4 and paxillin WT cells were transiently transfected with either Myc-tagged forms of dominant-negative Rac (N17 Rac) or dominant-negative Cdc42 (N17 Cdc42), detached and then allowed to spread on fibronectin for 60, 240, and 360 min. N17 Rac–transfected cells were then visualized by anti-Myc (a and b) and actin by RITC-phalloidin (c and d) and demonstrate that the introduction N17 Rac is able to completely inhibit the formation of multiple broad lamellipodia in paxillinΔLD4 cells. Images of the cells were captured at the 240-min time point and are representative of the differences in cell morphology observed at all time points. (C and D) Quantification of the ability of N17 Rac and N17 Cdc42 to abolish the morphological changes observed in paxillinΔLD4 cells demonstrates that both Rho family small GTPases affect the generation of multiple broad lamellipodia in paxillinΔLD4 cells. >200 cells were counted per time point, and values are the average of experiments performed in duplicate.
Figure 6.
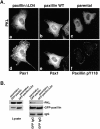
Deletion of paxillin LD4 affects endogenous PKL localization and paxillin–PKL association in vivo, but not FAK or vinculin localization or FAK activity. (A) PaxillinΔLD4, paxillin WT, and parental nontransfected CHO.K1 cells spread on fibronectin were processed for indirect immunofluorescence using antipaxillin antisera (Pax1) (d and e), phosphospecific Y118 paxillin anti-sera (f), and anti-PKL antibodies (a–c), which demonstrates the colocalization of paxillin and endogenous PKL in focal contacts in paxillin WT and parental nontransfected cells (b and e, c and f). In contrast, in paxillinΔLD4 cells, ectopic paxillin was found in focal contacts, whereas the endogenous PKL distribution was cytoplasmic (a and d, respectively). (B) Parental CHO.K1 cells were transiently transfected with either GFP–paxillin or GFP–paxillinΔLD4, detached from tissue culture dishes, and respread on fibronectin for 60 min. Coimmunoprecipitation assays were then performed followed by Western blot analysis and demonstrate that, although GFP–paxillin is capable of precipitating endogenous PKL, GFP–paxillinΔLD4 does not. (C) PaxillinΔLD4 cells spread on fibronectin were processed for indirect immunofluorescence using either anti-FAK (c), or antivinculin antibodies (d) and antipaxillin antisera (Pax1) (a and b). Arrowheads in a and b and arrows in c and d indicate that the deletion of paxillin LD4 does not affect the localization of these other paxillin-binding partners. (D) Parental nontransfected CHO.K1, paxillin WT, and paxillinΔLD4 cells were spread on fibronectin for 0, 20, and 120 min, and FAK was immunoprecipitated from lysates derived from these cells. Immunoblot analysis of cell lysates with antiphosphotyrosine antibody and of FAK immunoprecipitates with phosphospecific Y397 FAK antisera demonstrates equivalent FAK activation in the three cell lines tested.
Figure 6.
Deletion of paxillin LD4 affects endogenous PKL localization and paxillin–PKL association in vivo, but not FAK or vinculin localization or FAK activity. (A) PaxillinΔLD4, paxillin WT, and parental nontransfected CHO.K1 cells spread on fibronectin were processed for indirect immunofluorescence using antipaxillin antisera (Pax1) (d and e), phosphospecific Y118 paxillin anti-sera (f), and anti-PKL antibodies (a–c), which demonstrates the colocalization of paxillin and endogenous PKL in focal contacts in paxillin WT and parental nontransfected cells (b and e, c and f). In contrast, in paxillinΔLD4 cells, ectopic paxillin was found in focal contacts, whereas the endogenous PKL distribution was cytoplasmic (a and d, respectively). (B) Parental CHO.K1 cells were transiently transfected with either GFP–paxillin or GFP–paxillinΔLD4, detached from tissue culture dishes, and respread on fibronectin for 60 min. Coimmunoprecipitation assays were then performed followed by Western blot analysis and demonstrate that, although GFP–paxillin is capable of precipitating endogenous PKL, GFP–paxillinΔLD4 does not. (C) PaxillinΔLD4 cells spread on fibronectin were processed for indirect immunofluorescence using either anti-FAK (c), or antivinculin antibodies (d) and antipaxillin antisera (Pax1) (a and b). Arrowheads in a and b and arrows in c and d indicate that the deletion of paxillin LD4 does not affect the localization of these other paxillin-binding partners. (D) Parental nontransfected CHO.K1, paxillin WT, and paxillinΔLD4 cells were spread on fibronectin for 0, 20, and 120 min, and FAK was immunoprecipitated from lysates derived from these cells. Immunoblot analysis of cell lysates with antiphosphotyrosine antibody and of FAK immunoprecipitates with phosphospecific Y397 FAK antisera demonstrates equivalent FAK activation in the three cell lines tested.
Figure 7.
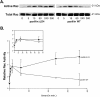
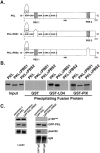
Characterization of the paxillin-binding site on PKL. (A) Schematic representations of full-length wild-type (PKL), deletion of PBS1 (PKLΔPBS1), and deletion of PBS2 (PKLΔPBS2) PKL proteins, respectively. (B) Radiolabeled wild-type PKL proteins and PKL protein containing either a deletion of PBS1 or PBS2 were produced by transcription/translation-coupled reactions and used in in vitro binding assays with GST–PIX or GST–LD4 to identify PBS2 as the paxillin-binding site on PKL. PIX binding to PKL was unaffected by either deletion. (C) Parental CHO.K1 cells were transiently transfected with either GFP–PKL or GFP–PKLΔPBS2, detached from tissue culture dishes, and respread on fibronectin for 60 min. Coimmunoprecipitation assays were then performed followed by Western blot analysis and demonstrate that GFP–PKL is capable of associating with paxillin in vivo, whereas GFP–PKLΔPBS2 is not. Immunoblot analysis with an antibody to p130cas was used to demonstrate the specificity of PKL–paxillin interaction.
Figure 8.
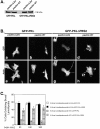
Introduction of PKLΔPBS2 mutant recapitulates the paxillinΔLD4 phenotype in paxillin wild-type cells. (A) Western blot analysis using GFP polyclonal antisera was used to confirm the presence of the ectopic GFP–PKL and PKLΔPBS2 after transient transfection of these constructs into paxillinΔLD4 and paxillin WT cells. (B) PaxillinΔLD4 and paxillin WT cells were transiently transfected separately with GFP–PKL or GFP–PKLΔPBS2, detached, and subjected to spreading assays on fibronectin for 60, 240, and 360 min. Transfected cells were then visualized by GFP fluorescence (a–d) and actin by RITC-phalloidin (e–h) and demonstrate that the introduction of PKLΔPBS2 into paxillin WT cells causes a transition to a paxillinΔLD4-like morphology. Arrow and arrowhead in f and h, respectively, demonstrate the lack of effect GFP–PKL has on paxillin WT cells, compared with the generation of broad lamellipodia induced by introduction of GFP–PKLΔPBS2. The double arrow in h indicates the presence of retraction fiber–like extensions in paxillin WT cells expressing GFP–PKLΔPBS2, similar to those observed in paxillinΔLD4 cells. Images of the cells were taken from the 240-min time point and are representative of the differences in cell morphology observed at all time points. (C) Quantification of morphological change was assessed by counting >200 cells per time point and demonstrate that the introduction of GFP–PKLΔPBS2 into paxillin WT cells recapitulates the paxillinΔLD4 phenotype, whereas GFP–PKL has relatively no affect. Values are the average of experiments performed in triplicate.
Figure 9.
Introduction of PKL and PKLΔPBS2 mutants does not affect paxillin localization to focal contacts, but PKLΔPBS2 does affect cell protrusiveness. (A) PaxillinΔLD4 and paxillin WT cells were transiently transfected separately with GFP–PKL or GFP–PKLΔPBS2, detached, and subjected to spreading assays on fibronectin for 60, 240, and 360 min. Transfected cells were then visualized by GFP fluorescence (a–d) and ectopic paxillin by Pax1 immunofluorescence (e–h). Immunofluorescence analysis demonstrates that the introduction of GFP–PKL and GFP–PKLΔPBS2 into paxillinΔLD4 and paxillin WT cells does not affect paxillin localization to focal contacts. Images of the cells were captured at the 240-min time point and are representative of the differences in cell morphology observed at all time points. (B) Quantification of protrusiveness demonstrates an increase in cell area (μm2) of CHO.K1 cells transfected with PKLΔPBS2 (left), as compared with parental nontransfected cells (Fig. 3 B, bottom). This observed increase in cell area is elevated by the cointroduction of paxillin and PKLΔPBS2 (right).
Similar articles
- Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness.
Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Brown MC, et al. Mol Biol Cell. 2005 Sep;16(9):4316-28. doi: 10.1091/mbc.e05-02-0131. Epub 2005 Jul 6. Mol Biol Cell. 2005. PMID: 16000375 Free PMC article. - Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway.
Brown MC, West KA, Turner CE. Brown MC, et al. Mol Biol Cell. 2002 May;13(5):1550-65. doi: 10.1091/mbc.02-02-0015. Mol Biol Cell. 2002. PMID: 12006652 Free PMC article. - Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling.
Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS. Turner CE, et al. J Cell Biol. 1999 May 17;145(4):851-63. doi: 10.1083/jcb.145.4.851. J Cell Biol. 1999. PMID: 10330411 Free PMC article. - Paxillin interactions.
Turner CE. Turner CE. J Cell Sci. 2000 Dec;113 Pt 23:4139-40. doi: 10.1242/jcs.113.23.4139. J Cell Sci. 2000. PMID: 11069756 Review. - Paxillin-ARF GAP signaling and the cytoskeleton.
Turner CE, West KA, Brown MC. Turner CE, et al. Curr Opin Cell Biol. 2001 Oct;13(5):593-9. doi: 10.1016/s0955-0674(00)00256-8. Curr Opin Cell Biol. 2001. PMID: 11544028 Review.
Cited by
- Paxillin mediates sensing of physical cues and regulates directional cell motility by controlling lamellipodia positioning.
Sero JE, Thodeti CK, Mammoto A, Bakal C, Thomas S, Ingber DE. Sero JE, et al. PLoS One. 2011;6(12):e28303. doi: 10.1371/journal.pone.0028303. Epub 2011 Dec 14. PLoS One. 2011. PMID: 22194823 Free PMC article. - Densification, phase stability and in vitro biocompatibility property of hydroxyapatite-10 wt% silver composites.
Nath S, Kalmodia S, Basu B. Nath S, et al. J Mater Sci Mater Med. 2010 Apr;21(4):1273-87. doi: 10.1007/s10856-009-3939-2. Epub 2009 Dec 5. J Mater Sci Mater Med. 2010. PMID: 19967432 - Feeling Things Out: Bidirectional Signaling of the Cell-ECM Interface, Implications in the Mechanobiology of Cell Spreading, Migration, Proliferation, and Differentiation.
Miller AE, Hu P, Barker TH. Miller AE, et al. Adv Healthc Mater. 2020 Apr;9(8):e1901445. doi: 10.1002/adhm.201901445. Epub 2020 Feb 9. Adv Healthc Mater. 2020. PMID: 32037719 Free PMC article. Review. - Crude aqueous extracts of Pluchea indica (L.) Less. inhibit proliferation and migration of cancer cells through induction of p53-dependent cell death.
Cho JJ, Cho CL, Kao CL, Chen CM, Tseng CN, Lee YZ, Liao LJ, Hong YR. Cho JJ, et al. BMC Complement Altern Med. 2012 Dec 26;12:265. doi: 10.1186/1472-6882-12-265. BMC Complement Altern Med. 2012. PMID: 23268709 Free PMC article. - Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness.
Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Brown MC, et al. Mol Biol Cell. 2005 Sep;16(9):4316-28. doi: 10.1091/mbc.e05-02-0131. Epub 2005 Jul 6. Mol Biol Cell. 2005. PMID: 16000375 Free PMC article.
References
- Arthur, W.T., L.A. Petch, and K. Burridge. 2000. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10:719–722. - PubMed
- Bagrodia, S., S.J. Taylor, K.A. Jordon, L. Van Aelst, and R.A. Cerione. 1998. A novel regulator of p21-activated kinases. J. Biol. Chem. 273:23633–23636. - PubMed
- Bokoch, G.M., and U.G. Knaus. 1994. Ras-related GTP-binding proteins and leukocyte signal transduction. Curr. Opin. Hematol. 1:53–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM047607/GM/NIGMS NIH HHS/United States
- R01 GM023244/GM/NIGMS NIH HHS/United States
- GM 47607/GM/NIGMS NIH HHS/United States
- GM23244/GM/NIGMS NIH HHS/United States
- R37 GM023244/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous