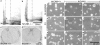Targeted ablation of NrCAM or ankyrin-B results in disorganized lens fibers leading to cataract formation - PubMed (original) (raw)
Targeted ablation of NrCAM or ankyrin-B results in disorganized lens fibers leading to cataract formation
M I Moré et al. J Cell Biol. 2001.
Abstract
The NgCAM-related cell adhesion molecule (NrCAM) is an immunoglobulin superfamily member of the L1 subgroup that interacts intracellularly with ankyrins. We reveal that the absence of NrCAM causes the formation of mature cataracts in the mouse, whereas significant pathfinding errors of commissural axons at the midline of the spinal cord or of proprioceptive axon collaterals are not detected. Cataracts, the most common cause of visual impairment, are generated in NrCAM-deficient mice by a disorganization of lens fibers, followed by cellular disintegration and accumulation of cellular debris. The disorganization of fiber cells becomes histologically distinct during late embryonic development and includes abnormalities of the cytoskeleton and of connexin50-containing gap junctions. Furthermore, analysis of lenses of ankyrin-B mutant mice also reveals a disorganization of lens fibers at postnatal day 1, indistinguishable from that generated by the absence of NrCAM, indicating that NrCAM and ankyrin-B are required to maintain contact between lens fiber cells. Also, these studies provide genetic evidence of an interaction between NrCAM and ankyrin-B.
Figures
Figure 1.
NrCAM gene disruption. (a) Map of the targeting vector, and of part of the genomic locus before and after homologous recombination with the targeting vector. The position of exons 5, 6, and 8 as well as the exon numbering was adopted from the human NrCAM locus (Dry et al., 2001). neo, PGKneopA cassette; tk, MC1TKpA cassette; H, HindIII; K, KpnI; S, SacI; P, PauI; B, BamHI; N, NheI; Sp, SpeI. Only relevant restriction sites are shown. The positions of the external Southern blot probe, as well as the PCR primers, are indicated. (b) Southern blot of DNA from tail biopsies of wild-type, heterozygous, and homozygous mice, using the restriction enzymes BamHI and KpnI. (c) PCR of genomic DNA using the primers Mimo71 and Moré1 (c), and the primers neoL1 and Moré1. (d) Immunoblot blot analysis using whole brain from adult wild-type and NrCAM−/− mice using polyclonal antibody 463 against NrCAM. The NrCAM band is completely absent in the mutants. Additional bands are due to nonspecific binding activity of the antibody. To demonstrate equal loading, a parallel blot was analyzed with polyclonal antibodies to L1. Molecular weight markers are indicated on the left. Immunohistological studies using neural tissue as well as immunoblots of brain and lens tissue using a polyclonal antibody provided by M. Grumet also reveal that NrCAM is absent (not shown).
Figure 2.
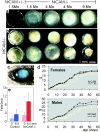
NrCAM-deficient mice develop cataracts and reveal reduced body weight and motor disabilities. (a) Stages of cataract development. From left to right: 1.5-mo-old NrCAM+/− lens; NrCAM−/− littermate; both lenses from 4-mo-old NrCAM−/− mouse; 9-mo-old NrCAM−/− lens; same magnification as in b. (b) Lens pairs from 11-mo-old NrCAM−/− siblings. (c) Eye of a freshly killed 4-mo-old NrCAM mouse. (d) Weights of NrCAM+/− and NrCAM−/− littermates during postnatal development. Black line and symbols, NrCAM+/− animals; light line and symbols, NrCAM−/− animals. (e) Rotarod test. The number of times the animals fell from the rod in 3 min is indicated for a nonmoving rod and the rod rotating with 6.5 rpm. Each animal was confronted without prior rod experience, first with the nonmoving rod, and after a recovery time, with the rotating rod.
Figure 3.
Expression of NrCAM in lens. (a) Schematic overview of an adult mouse lens as central longitudinal section. The lens has a spheric shape with a symmetry around its rotational axis. It contains primary lens fibers that are formed during early embryonic development and secondary lens fibers that are formed during later embryonic development and throughout life. The anterior end (pointing upwards) has a monolayer of epithelial cells that can divide and migrate towards the sides of the lens, followed by an elongation and maturation process, in which the nucleus and other organelles are lost and characteristics of secondary fiber cells are adopted. For clarity, the capsule, epithelial cells, and lens fibers are not drawn to scale (adapted from Bassnett et al., 1999). (b) Immunoblot of mouse lens proteins using 463 polyclonal antibody against NrCAM. Proteins of lenses from animals aged as indicated are loaded. (c–e) Same magnification. (c) Noncentral longitudinal section through 9-mo-old wild-type mouse secondary lens fibers stained with polyclonal antibodies against NrCAM. (d) E14 chick noncentral longitudinal lens section stained with monoclonal antibody number 3 against chick NrCAM. (e) E14 chick lens longitudinal section stained with polyclonal antibodies against NrCAM. (f and g) Mouse P12 noncentral longitudinal lens sections stained with polyclonal antibodies against NrCAM. ca., capsule; ep., epithelium. (f) side-front region with epithel. The outer edge of the capsule is marked. (g) Posterior edge of the lens. (h and i) Immunoblots of E14 brain (B) and lens (L) lysates reveal that only NrCAM, but no other family members or extracellular NrCAM interaction partner is present in the lens. Since antibodies to anti-CHL1 or anti-RPTPβ/ζ are available only for mouse proteins, these were not tested in chick. For the other proteins, the chick immunoblot is shown for reasons of availability or quality of the antibodies. Molecular weight markers are indicated on the left. (h) Expression of NrCAM, NrCAM homologues NgCAM (species homologue of mouse L1), neurofascin, and CHL1. Note that neurofascin is expressed in several molecular weight forms. (i) Expression of extracellular NrCAM interaction partners F11, axonin-1, and RPTPβ/ζ.
Figure 4.
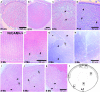
Cataract histology of NrCAM-deficient mice. From the emergence of secondary lens fibers onwards, NrCAM-deficient lens fibers develop a rounded instead of elongated shape, indicating loss of cell–cell contact. The phenotype is progressive and leads to a disintegration of lens fibers and accumulation of cellular debris. (a) NrCAM−/− E14.5 longitudinal section, HE stained. (b and c) NrCAM−/− E18 longitudinal sections, HE stained. (d) NrCAM−/− P12 noncentral longitudinal section, anterior region, TB stained. (c–k) Positions of sections in the lens are indicated in l. (e) 2-mo-old wild-type posterior region in noncentral longitudinal section, HE stained. (f) Same as e, but with NrCAM−/−. (g–h) 2-mo-old NrCAM−/− central longitudinal section, TB stained. (g) One-third of the way to the center side-front region. (h) Half-way to the center posterior region. (i–k) 8-mo-old NrCAM−/− lens in longitudinal noncentral section, HE stained. (i) Marginal region. (j) Anterior region. (k) Posterior region. *Cellular debris; arrowheads, intensely stained rounded cell; arrows, examples of secondary fiber cells in the process of rounding up due to contact loss of neighboring cells; open arrows, cells in the process of disintegration.
Figure 5.
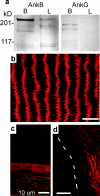
Ankyrin-B but not -G is expressed in the lens. (a) Immunoblot of brain and lens lysates using antibodies against ankyrin-B or -G. The molecular weight markers are indicated on the left. (b) Longitudinal noncentral section of 9-mo-old mouse lens stained with antibodies against ankyrin-B. (c) Same as b, but anterior region showing expression of ankyrin-B in the epithelium but not the capsule. (d) P12 longitudinal lens section showing that the lens fiber membranes neighboring the capsule do not express ankyrin-B. The capsule position is indicated.
Figure 6.
The absence of ankyrin-B causes similar lens fiber disorganization as the absence of NrCAM. (a and b) Longitudinal section through a P0.5 ankyrin-B−/− lens, HE stained. Arrows, rounded abnormal shaped cells. (c and d) Longitudinal section through a P0.5 ankyrin-B+/+ sibling lens, HE stained. (e) Longitudinal section through a P1 ankyrin-B−/− lens, stained with the F-actin stain FITC-phalloidin. Arrows, aggregates of F-actin.
Figure 7.
**Disorganization of F-actin and connexin50 in the absence of NrCAM. (**a and b) P12 and 2.5-mo-old, respectively, lateral-anterior longitudinal section through an NrCAM−/− lens, stained with FITC-phalloidin. Filled arrowheads, examples of F-actin aggregates; open arrowheads, examples of bordering cell membranes with a still normal ordered F-actin arrangement. (c and d) 1-mo-old lenses stained with monoclonal antibodies against the gap junctional protein connexin50. (c) wild type. (d) NrCAM−/−. Arrows, con-nexin50 aggregates.
Figure 8.
Analysis of axonal pathfinding and neurite extension. In NrCAM-deficient mice, commissural axons cross the spinal cord midline (floor plate) as efficiently as in wild type. Rostral is up. (a and b) Open book preparations of DiI tracing of E12.5 commissural axons. (a) Heterozygote. (b) NrCAM−/−. The broken lines indicate the location of the floor plates. (c and d) Immunostains of E15 proprioceptive neurons in forelimb region of the spinal cord using antibodies against parvalbumin reveal no differences in the pathfinding of proprioceptive collaterals. (c) Wild-type. (d) NrCAM−/−. (e–m) 24-h neurite outgrowth of whole brain embryonal neurons. Unlike wild type, NrCAM-deficient neurons are unable to grow on the subtrates F11 or neurofascin (NF). However, they can grow on the complexes F11–tenascin-R or NF–tenascin-R (TN-R), since instead of NrCAM β1 integrins and at least one additional protein are used as receptors. (e, h, and k) E12 wild-type neurons. (f, g, i, j, l, and m) NrCAM−/− neurons. (e and f) No substrate. (g) Surface blocked with BSA, followed by incubation with TN-R before neuron seeding. (h and i) F11 as substrate. (j) F11 as substrate, then BSA blockage and TN-R treatment. (k and l) Neurofascin-Fc as substrate. (m) Neurofascin-Fc as substrate, then BSA blockage and TN-R treatment.
Similar articles
- Connections between connexins, calcium, and cataracts in the lens.
Gao J, Sun X, Martinez-Wittinghan FJ, Gong X, White TW, Mathias RT. Gao J, et al. J Gen Physiol. 2004 Oct;124(4):289-300. doi: 10.1085/jgp.200409121. J Gen Physiol. 2004. PMID: 15452195 Free PMC article. - Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels.
Bennett V, Lambert S. Bennett V, et al. J Neurocytol. 1999 Apr-May;28(4-5):303-18. doi: 10.1023/a:1007005528505. J Neurocytol. 1999. PMID: 10739573 Review. - Physiological and Optical Alterations Precede the Appearance of Cataracts in Cx46fs380 Mice.
Minogue PJ, Gao J, Zoltoski RK, Novak LA, Mathias RT, Beyer EC, Berthoud VM. Minogue PJ, et al. Invest Ophthalmol Vis Sci. 2017 Aug 1;58(10):4366–4374. doi: 10.1167/iovs.17-21684. Invest Ophthalmol Vis Sci. 2017. PMID: 28810266 Free PMC article. - Neuron glia-related cell adhesion molecule (NrCAM) promotes topographic retinocollicular mapping.
Dai J, Buhusi M, Demyanenko GP, Brennaman LH, Hruska M, Dalva MB, Maness PF. Dai J, et al. PLoS One. 2013 Sep 2;8(9):e73000. doi: 10.1371/journal.pone.0073000. eCollection 2013. PLoS One. 2013. PMID: 24023801 Free PMC article. - Connexin Mutants Compromise the Lens Circulation and Cause Cataracts through Biomineralization.
Berthoud VM, Gao J, Minogue PJ, Jara O, Mathias RT, Beyer EC. Berthoud VM, et al. Int J Mol Sci. 2020 Aug 13;21(16):5822. doi: 10.3390/ijms21165822. Int J Mol Sci. 2020. PMID: 32823750 Free PMC article. Review.
Cited by
- Mouse models of cataract.
Graw J. Graw J. J Genet. 2009 Dec;88(4):469-86. doi: 10.1007/s12041-009-0066-2. J Genet. 2009. PMID: 20090208 Review. - Ankyrin-G regulated epithelial phenotype is required for mouse lens morphogenesis and growth.
Rasiah PK, Maddala R, Bennett V, Rao PV. Rasiah PK, et al. Dev Biol. 2019 Feb 1;446(1):119-131. doi: 10.1016/j.ydbio.2018.12.016. Epub 2018 Dec 15. Dev Biol. 2019. PMID: 30562487 Free PMC article. - Calpain expression and activity during lens fiber cell differentiation.
De Maria A, Shi Y, Kumar NM, Bassnett S. De Maria A, et al. J Biol Chem. 2009 May 15;284(20):13542-13550. doi: 10.1074/jbc.M900561200. Epub 2009 Mar 6. J Biol Chem. 2009. PMID: 19269960 Free PMC article. - Ankyrin-B directs membrane tethering of periaxin and is required for maintenance of lens fiber cell hexagonal shape and mechanics.
Maddala R, Walters M, Brophy PJ, Bennett V, Rao PV. Maddala R, et al. Am J Physiol Cell Physiol. 2016 Jan 15;310(2):C115-26. doi: 10.1152/ajpcell.00111.2015. Epub 2015 Nov 4. Am J Physiol Cell Physiol. 2016. PMID: 26538089 Free PMC article. - Molecular characterization of mouse lens epithelial cell lines and their suitability to study RNA granules and cataract associated genes.
Terrell AM, Anand D, Smith SF, Dang CA, Waters SM, Pathania M, Beebe DC, Lachke SA. Terrell AM, et al. Exp Eye Res. 2015 Feb;131:42-55. doi: 10.1016/j.exer.2014.12.011. Epub 2014 Dec 19. Exp Eye Res. 2015. PMID: 25530357 Free PMC article.
References
- Bassnett, S., H. Missey, and I. Vucemilo. 1999. Molecular architecture of the lens fiber cell basal membrane complex. J. Cell Sci. 112:2155–2165. - PubMed
- Berry, V., P. Francis, S. Kaushal, A. Moore, and S. Bhattacharya. 2000. Missense mutations in MIP underlie autosomal dominant ‘polymorphic’ and lamellar cataracts linked to 12q. Nat. Genet. 25:15–17. - PubMed
- Brümmendorf, T., and F.G. Rathjen. 1995. Cell adhesion molecules 1: immunoglobulin superfamily. Prot. Profile. 2:963–1108. - PubMed
- Brümmendorf, T., S. Kenwrick, and F.G. Rathjen. 1998. Neural cell recognition molecule L1: from cell biology to human hereditary brain malformations. Curr. Opin. Neurobiol. 8:87–97. - PubMed
- Burmeister, M., Q. Ren, G.J. Makris, D. Samson, and V. Bennett. 1996. Genes for the neuronal immunoglobulin domain cell adhesion molecules neurofascin and Nr-CAM map to mouse chromosomes 1 and 12 and homologous human chromosomes. Mamm. Genome. 7:558–559. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous