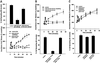Modulation of cell-substrate adhesion by arachidonic acid: lipoxygenase regulates cell spreading and ERK1/2-inducible cyclooxygenase regulates cell migration in NIH-3T3 fibroblasts - PubMed (original) (raw)
Modulation of cell-substrate adhesion by arachidonic acid: lipoxygenase regulates cell spreading and ERK1/2-inducible cyclooxygenase regulates cell migration in NIH-3T3 fibroblasts
R A Stockton et al. Mol Biol Cell. 2001 Jul.
Free PMC article
Abstract
Adhesion of cells to an extracellular matrix is characterized by several discrete morphological and functional stages beginning with cell-substrate attachment, followed by cell spreading, migration, and immobilization. We find that although arachidonic acid release is rate-limiting in the overall process of adhesion, its oxidation by lipoxygenase and cyclooxygenases regulates, respectively, the cell spreading and cell migration stages. During the adhesion of NIH-3T3 cells to fibronectin, two functionally and kinetically distinct phases of arachidonic acid release take place. An initial transient arachidonate release occurs during cell attachment to fibronectin, and is sufficient to signal the cell spreading stage after its oxidation by 5-lipoxygenase to leukotrienes. A later sustained arachidonate release occurs during and after spreading, and signals the subsequent migration stage through its oxidation to prostaglandins by newly synthesized cyclooxygenase-2. In signaling migration, constitutively expressed cyclooxygenase-1 appears to contribute approximately 25% of prostaglandins synthesized compared with the inducible cyclooxygenase-2. Both the second sustained arachidonate release, and cyclooxygenase-2 protein induction and synthesis, appear to be regulated by the mitogen-activated protein kinase extracellular signal-regulated kinase (ERK)1/2. The initial cell attachment-induced transient arachidonic acid release that signals spreading through lipoxygenase oxidation is not sensitive to ERK1/2 inhibition by PD98059, whereas PD98059 produces both a reduction in the larger second arachidonate release and a blockade of induced cyclooxygenase-2 protein expression with concomitant reduction of prostaglandin synthesis. The second arachidonate release, and cyclooxygenase-2 expression and activity, both appear to be required for cell migration but not for the preceding stages of attachment and spreading. These data suggest a bifurcation in the arachidonic acid adhesion-signaling pathway, wherein lipoxygenase oxidation generates leukotriene metabolites regulating the spreading stage of cell adhesion, whereas ERK 1/2-induced cyclooxygenase synthesis results in oxidation of a later release, generating prostaglandin metabolites regulating the later migration stage.
Figures
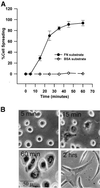
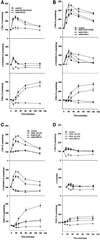
Figure 1
Kinetics and morphology of NIH-3T3 cell spreading on fibronectin. (A) Percentage of cells spread versus time. Detached cells were suspended in serum-free medium and plated on plastic dishes coated with 20 μg of FN or 50 μg of BSA. Percentage of spreading was determined as described in MATERIALS AND METHODS. Points with error bars represent means with SEs (n = 4). (B) Morphology of cell spreading over time on FN. Under the conditions used herein, cells attached to fibronectin but remain rounded within 5 min of plating (5 min). Partial spreading is observed commencing at ∼15 min (15 min); full spreading of 90–95% of the cell population is seen at ∼60 min (60 min). The typically fibroblast pyramidal shape seen 2 h postplating appears to be the migratory phenotype in these cells (2 h).
Figure 2
(A) 3T3 cell spreading is sensitive to inhibition of a phospholipase A2. Detached cells were suspended in serum-free medium with or without the indicated inhibitor for 15 min and then plated on a FN substrate. Percentage of spreading was evaluated at 60 min. Bars indicate treatment with 30 μM mepacrine [inhib all PLA2 (M)] or 3 μM BPB [inhib all PLA2 (B)], general inhibitors of all PLA2 isoforms; or with AACOCF3 (25 μM), an inhibitor of both iPLA2 and cPLA2 (inhib cPLA2 + iPLA2), or with the iPLA2-specific inhibitor HELSS (10 μM) (inhib iPLA2). All are shown either with (black bars) or without (white bars) 15 μM arachidonic acid (n = 3; means with SEs shown). Mepacrine, BPB, and AACOCF3 all produce dose-dependent spreading inhibition; a single higher concentration of each is shown herein that is reversible with exogenous AA. HELSS treatment produced a small 10–15% reduction at any concentration but the effect was not reversible by addition of AA.
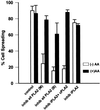
Figure 3
(A) Leukotriene B4 reverses spreading blockade from inhibitors of 5-lipoxygenase or cytosolic phospholipase A2. General inhibition of lipoxygenases with 30 μM NDGA (inhib all LOX), and specific inhibition of 5-LOX with 25 μM AA861 (inhib 5-LOX) reduced NIH-3T3 cell spreading, whereas inhibition of either 12-LOX with up to 100 μM baicalein (inhib 12-LOX), or of 15-LOX with up to 25 μM PD146176 (inhib 15-LOX) had no effect. The decrease from NDGA or AA861 was dose-dependent; representative higher concentrations are shown herein. The spreading blockade due to inhibition of any LOX by NDGA or 5-LOX by AA861, or of cPLA2 and iPLA2 by AACOCF3 (inhib iPLA2 + cPLA2) was reversible by addition of the 5-LOX oxidative product 100 nM LTB4 (black bars). Detached cells were incubated with indicated treatment and then plated on FN-coated dishes for 60 min at 37°C (n = 3; means with SEs). (B) Exogenous prostaglandin E2 does not reverse spreading blockade due to phospholipase A2 or lipoxygenase inhibitors. Cells were treated with inhibitors and allowed to spread as in A, except with the cyclooxygenase oxidative product PGE2, at 50 nM (n = 2).
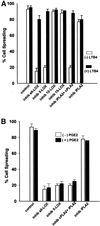
Figure 4
(A) Cyclooxygenase, epoxygenase, and ERK activity are not required for cell spreading. Cells were detached, suspended in serum-free medium, and incubated with varying concentrations of COX, EOX, or ERK1/2/inhibitors before plating on FN-coated plates at 37°C. Single representative higher concentrations are shown herein: 50 μM indomethacin against both cyclooxygenases (inhib COX1 + COX2), 25 μM resveratrol against COX-1 (inhib COX-1), 25 μM SC236 against COX-2 (inhib COX-2), 50 μM metyrapone against epoxygenase (inhib EOX), or 50 μM PD98059 against ERK1/2 (inhib ERK1/2). Percentage of cell spreading was evaluated at 60 min (bars indicate means and SE; n = 3). (B) Mean spread cell surface area at 60 min is increased by exogenous arachidonic acid or leukotriene B4, and by cyclooxygenase-1/2 or ERK1/2 inhibitors. Cells treated as in A were photographed at the same magnification and mean cell surface area measured by SigmaScanPro surface area analysis program. Bars are labeled as in A and indicate means and SE normalized to percentage of control surface area. Exogenous PGE2 (50 nM) reverses the spreading cell surface area enhancement seen with addition of AA, LTB4, indomethacin, resveratrol, SC236, or PD98059 at concentrations shown in A. Addition of 50 nM PGE2 alone reduced surface area at 60 min by ∼10% compared with control untreated cells (n = 200 cells/treatment/field × three fields). (C) Morphology of spreading cells with arachidonic acid or leukotriene B4, and with lipoxygenase, cyclooxygenase or ERK inhibitors. Representative cell spreading at 60 min shown with or without 50 μM PD98059 to block ERK1/2 (inhib ERK), 50 μM indomethacin to block both COX-1 and COX-2 (inhib COX-1 and -2), 25 μM SC236 to block COX-2 (inhib COX-2), or 25 μM resveratrol to inhibit COX-1 (inhib COX-1) at concentrations indicated in A. The enhancement of spread cell surface area induced by these inhibitors resembles those of cells treated with 15 μM AA alone (+AA), or with 50 nM LTB4 (+LTB4), an oxidative product of 5-LOX, whereas addition of 50 nM PGE2 (+PGE2) slightly reduces surface area and alters overall shape. (D) Cell spreading kinetic rate is also increased by cyclooxygenase inhibitors. Cells treated with the same inhibitors at the concentrations as in A were measured for the rate of cell spreading over time as described in Figure 1A. COX inhibitors increased cell spreading rate over the first 30 min of spreading in a dose-dependent manner; representative higher concentrations are shown herein (means and SEs; n = 4).
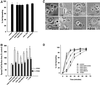
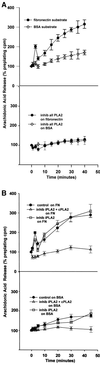
Figure 5
(A) NIH-3T3 cell spreading on a fibronectin substrate produces a biphasic release of arachidonic acid from phospholipase A2 activity. (Top) Cells were incubated in 4 μM [3H]arachidonic acid for 18 h, detached, suspended in serum-free medium, and plated on either a FN (black circles) or a BSA (open diamonds) substrate. Cells spread fully on FN but do not attach or spread on BSA. Samples of medium were removed at the indicated intervals, and AA evaluated as appearance of label in the medium. Data are normalized as a percentage of [3H]AA counts in the medium before plating (means with SE; n = 4). (Bottom) [3H]AA release from cells treated with 30 μM mepacrine, an inhibitor of all PLA2s, before plating on either substrate. (B) Treatment of cells with 25 μM AACOCF3 (open diamonds), an inhibitor of both iPLA2 and cPLA2, also reduces AA release from cells plated on a FN or BSA substrate, whereas treatment with 10 μM HELSS (open squares), a specific inhibitor of iPLA2 only, does not block AA release (n = 3).
Figure 6
(A) Initial transient arachidonic acid release signals most cell spreading. The general PLA2 inhibitor mepacrine (30 μM), added to plated cells at 5 min postplating after the early transient AA release seen in Figure 5A had already occurred, suppressed the second AA release but not the first (bottom; open squares) and still permitted most cell spreading (top; inhib all PLA2 5 min post-plating). Treatment with the same inhibitor before plating suppresses both phases of AA release (bottom; open triangles), and cell spreading (top; inhib all PLA2 pre-plating). (B, top) As with mepacrine in A, 25 μM AACOCF3 (inhib iPLA2 + cPLA2), added to cells after the initial transient AA release at 5 min, blocks the second AA release but not the first, and still permits most cell spreading (bottom) (means and SEs; n = 3). (C) HELSS (10 μM) (inhib iPLA2) added to cells either at 5 min after the initial AA release, or incubated before plating, does not significantly block either phase of AA release (top), or cell spreading (bottom).
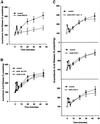
Figure 7
(A) Inhibition of ERK1/2 phosphorylation with PD98059 partially suppresses the second, but not the initial transient arachidonic acid release, during cell spreading. Cells were treated with 50 μM PD98059 to inhibit activation of ERK1/2 as described in MATERIALS AND METHODS. Control untreated (closed circles) and ERK-inhibited (open triangles) cells respond similarly until 10–15 min postplating, at the beginning of the sustained second phase of AA release; ERK inhibition results in ∼50% reduction in the second AA release without blocking the first transient AA release (means and SEs; n = 3.), while not inhibiting cell spreading (Figure 4A). (B) 5-Lipoxygenase inhibition does not affect AA release. Cells treated with either the general LOX inhibitor NDGA at 30 μM (inhib all LOX) or the 5-LOX inhibitor AA861 (25 μM) (inhib 5-LOX) do not experience reduced AA release in either phase (n = 2). (C) Cyclooxygenase inhibition does not affect AA release. Cells treated with 50 μM indomethacin to inhibit COX-1 and -2 (inhib COX-1 and -2), 25 μM resveratrol to inhibit COX-1 (inhib COX-1), or 25 μM SC236 to inhibit COX-2 ([inhib COX-2] were tested for AA release during spreading as in A. (n = 3).
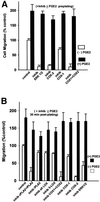
Figure 8
Cell migration requires ERK1/2 and cyclooxygenase activity. (A) NIH-3T3 cell migration was evaluated with Boyden chamber assays as described in MATERIALS AND METHODS. Cells were treated with 50 μM PD98059 to inhibit ERK1/2 (inhib ERK1/2), 50 μM indomethacin to inhibit both cyclooxygenases (inhib COX1 + COX2), 25 μM SC236 to inhibit cyclooxygenase-2 (inhib COX-2), or 25 μM resveratrol to inhibit cyclooxygenase-1 (inhib COX-1). White bars indicate inhibitor alone, black bars indicate addition of the cyclooxygenase oxidation product PGE2 (50 nM) with inhibitor. Dose-dependent reduction in migration was seen with PD98059, indomethacin, and SC236, the figure shows a typical higher concentration of each reversible by exogenous PGE2. Resveratrol inhibition of COX-1 was dose-dependent for a limited range, but did not reduce migration to <70% of control at concentrations >25 μM (means and SEs; n = 3) (B) Inhibitors of COX-2 or PLA2 added after 30 min of cell spreading block most cell migration. Inhibitors were added to cells after 30 min of cell plating on FN after the initial AA release had taken place, to test the requirement for AA generated in the second sustained release as a substrate for cyclooxygenase activity. Addition of 25 μM AACOCF3 (inhib iPLA2 + cPLA2), 50 μM indomethacin (inhib COX1 + COX2), or 25 μM SC236 (inhib COX-2), after 30 min of cell spreading, reduces migration in a dose-dependent manner; a single representative inhibitory concentration is shown herein that is reversible by PGE2 addition. The COX-1 inhibitor resveratrol at 25 μM (inhib COX-1) producesonly ∼25% reduction in migration when added after 30 min of spreading. Inhibition of ERK1/2 with 50 μM PD98059 at 30 min postspreading also reduced migration only to ≥70% of control, compared with the migration of ≤15% of control seen in A at the same concentration added preplating. Post 30 min spreading addition of 10 μM HELSS (inhib iPLA2) produced a 10–15% reduction in migration that was not dose-dependent. Addition of 30 μM NDGA (inhib all LOX) or 25 μM AA861 (inhib 5-LOX) at 30 min postplating reduced migration also by only 15–20%.
Figure 9
Lipoxygenase and cyclooxygenase activity during cell adhesion to FN is modulated by the competing oxidative branch enzymes, and by ERK1/2. (A) Arachidonic acid produced by cPLA2, but not iPLA2, is the substrate for both LOX and COX oxidative activities. Cells were detached, incubated in serum-freemedium with either 25 μM AACOCF3 (inhib iPLA2 + cPLA2) or 10 μM HELSS (inhib iPLA2), and plated on FN-coated plates as described in MATERIALS AND METHODS. Cells plus medium were collected at indicated time points and assayed by enzymeimmunoassay for the 5-LOX oxidative products LTB4 (top) and cysteinyl leukotrienes LTC4/D4/E4 (middle), and for the cyclooxygenase product PGE2 (bottom). (B) Inhibition of cyclooxygenases increases 5-lipoxygenase activity. Cells were treated with or without 50 μM indomethacin (inhib COX1 + COX2), 25 μM resveratrol (inhib COX-1), or 25 μM SC236 (inhib COX-2), plated on FN-coated plates, and collected over time as in A. (C) Inhibition of 5-LOX blocks leukotriene synthesis but does not increase prostaglandin levels; inhibition of ERK1/2 blocks PGE2 synthesis and also increases leukotriene levels. Cells were treated with 50 μM PD98059 (inhib ERK1/2), 30 μM NDGA (inhib all LOX), or 25 μM AA861 (inhib 5-LOX) and spread on FN-coated plates before collection and immunoassay as described in MATERIALS AND METHODS. (D) Leukotriene and prostaglandin E2 synthesis is generally reduced in cells plated on a BSA substrate. Cells treated with 30 μM NDGA (inhib all LOX), 50 μM indomethacin (inhib all COX), or 50 μM PD98059 to prevent ERK1/2 activation (inhib ERK1/2), were plated on a BSA substrate where no spreading occurs, to compare with prostanoid synthesis on a FN substrate as seen in A–C (means and SEs; n = 3).
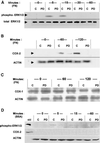
Figure 10
Inhibition of ERK1/2 activation blocks the induction and synthesis of COX-2 protein during NIH-3T3 cell adhesion. Cells with or without 50 μM PD98059 to inhibit activation of ERK1/2 were allowed to attach and spread on FN (A–C), or plated on BSA where no spreading occurs (D), for the indicated times in minutes. Cells were lysed and subjected to SDS-PAGE and immunoblotting as described in MATERIALS AND METHODS. In all blots, control untreated cell lanes are indicated with C, and cells treated with PD98059 are indicated with PD. (A) ERK1/2 expression and activation by phosphorylation during cell adhesion to FN. Total ERK1/2 as a loading indicator is shown in the bottom row, detected with anti-pan-ERK antibody (total ERK1/2). Activated ERK1/2 is shown in the top row, detected with anti-phosphoERK1/2 antibody (phospho-ERK1/2). (B) Inducible COX-2 expression during cell adhesion to FN. COX-2 protein was detected with an anti-COX-2 antibody (COX-2), and actin was probed (ACTIN) as a loading control. (C) COX-1 expression during cell adhesion to FN. COX-1 protein expression was probed with anti-COX-1 antibody (COX-1), with actin probed as a loading control (ACTIN). (D) ERK1/2 and COX-2 in nonspreading cells plated on BSA. The top blot strip was probed with anti-phosphoERK; a phospho-ERK protein standard is the upper left lane (std). The middle strip was probed for COX-2, also with a standard in the left lane. The bottom strip shows actin in the same cells. The blots shown are typical representatives of three experiments.
Figure 11
Model of a bifurcated arachidonic acid pathway regulating a transition from cell spreading to cell migration. NIH-3T3 cell spreading on fibronectin is by means of α5β1-integrin receptors in the plasma membrane initially binding extracellular matrix fibronectin. The signaling pathway begins with integrin receptor clustering during cell-ECM attachment, which stimulates rapid cPLA2 activation and an immediate transient AA release oxidized by 5-lipoxygenase, generating leukotrienes to signal cell spreading. ERK1/2 is also rapidly activated during the cell attachment phase, and modulates cPLA2 activity to enhance a later sustained AA release, as well as inducing the immediate expression of COX-2 protein. Up-regulation of COX-2 increases total prostaglandin production over that contributed by constitutive COX-1, to stimulate a postspreading transition to migration.
Similar articles
- 5-lipoxygenase and cyclooxygenase regulate wound closure in NIH/3T3 fibroblast monolayers.
Green JA, Stockton RA, Johnson C, Jacobson BS. Green JA, et al. Am J Physiol Cell Physiol. 2004 Aug;287(2):C373-83. doi: 10.1152/ajpcell.00509.2003. Epub 2004 Apr 7. Am J Physiol Cell Physiol. 2004. PMID: 15197007 - Elevated levels of cyclooxygenase-2 in antigen-stimulated mast cells is associated with minimal activation of p38 mitogen-activated protein kinase.
Hundley TR, Prasad AR, Beaven MA. Hundley TR, et al. J Immunol. 2001 Aug 1;167(3):1629-36. doi: 10.4049/jimmunol.167.3.1629. J Immunol. 2001. PMID: 11466386 - Affinities of various mammalian arachidonate lipoxygenases and cyclooxygenases for molecular oxygen as substrate.
Juránek I, Suzuki H, Yamamoto S. Juránek I, et al. Biochim Biophys Acta. 1999 Jan 4;1436(3):509-18. doi: 10.1016/s0005-2760(98)00159-3. Biochim Biophys Acta. 1999. PMID: 9989280 - Dual acting anti-inflammatory drugs: a reappraisal.
Bertolini A, Ottani A, Sandrini M. Bertolini A, et al. Pharmacol Res. 2001 Dec;44(6):437-50. doi: 10.1006/phrs.2001.0872. Pharmacol Res. 2001. PMID: 11735348 Review. - Identification of an endogenous inhibitor of arachidonate metabolism in human epidermoid carcinoma A431 cells.
Chang WC. Chang WC. J Biomed Sci. 2003;10(6 Pt 1):599-606. doi: 10.1159/000073525. J Biomed Sci. 2003. PMID: 14576462 Review.
Cited by
- Arachidonic acid and colorectal carcinogenesis.
Jones R, Adel-Alvarez LA, Alvarez OR, Broaddus R, Das S. Jones R, et al. Mol Cell Biochem. 2003 Nov;253(1-2):141-9. doi: 10.1023/a:1026060426569. Mol Cell Biochem. 2003. PMID: 14619964 Review. - A signalling cascade involving receptor-activated phospholipase A2, glycerophosphoinositol 4-phosphate, Shp1 and Src in the activation of cell motility.
Varone A, Mariggiò S, Patheja M, Maione V, Varriale A, Vessichelli M, Spano D, Formiggini F, Lo Monte M, Brancati N, Frucci M, Del Vecchio P, D'Auria S, Flagiello A, Iannuzzi C, Luini A, Pucci P, Banci L, Valente C, Corda D. Varone A, et al. Cell Commun Signal. 2019 Mar 1;17(1):20. doi: 10.1186/s12964-019-0329-3. Cell Commun Signal. 2019. PMID: 30823936 Free PMC article. - Cellular impedance biosensors for drug screening and toxin detection.
Asphahani F, Zhang M. Asphahani F, et al. Analyst. 2007 Sep;132(9):835-41. doi: 10.1039/b704513a. Epub 2007 Jun 26. Analyst. 2007. PMID: 17710258 Free PMC article. Review. - Blockade of TRPM7 channel activity and cell death by inhibitors of 5-lipoxygenase.
Chen HC, Xie J, Zhang Z, Su LT, Yue L, Runnels LW. Chen HC, et al. PLoS One. 2010 Jun 17;5(6):e11161. doi: 10.1371/journal.pone.0011161. PLoS One. 2010. PMID: 20567598 Free PMC article. - Fibroblast growth on micro- and nanopatterned surfaces prepared by a novel sol-gel phase separation method.
Reemann P, Kangur T, Pook M, Paalo M, Nurmis L, Kink I, Porosaar O, Kingo K, Vasar E, Kõks S, Jaks V, Järvekülg M. Reemann P, et al. J Mater Sci Mater Med. 2013 Mar;24(3):783-92. doi: 10.1007/s10856-012-4829-6. Epub 2012 Dec 13. J Mater Sci Mater Med. 2013. PMID: 23239263
References
- Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem. 1999;274:5038–5046. - PubMed
- Balsinde J, Balboa MA, Insel PA, Dennis EA. Regulation and inhibition of phospholipase A2. Annu Rev Pharmacol Toxicol. 1999;39:175–189. - PubMed
- Barry OP, Kazanietz MG, Pratico D, Fitzgerald GA. Arachidonic acid in platelet microparticles up-regulates cyclooxygenase-2-dependent prostaglandin formation via a protein kinase C/mitogen-activated protein kinase-dependent pathway. J Biol Chem. 1999;274:7545–7556. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous