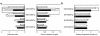Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice - PubMed (original) (raw)
I Kato, T Doi, H Yonekura, S Ohashi, M Takeuchi, T Watanabe, S Yamagishi, S Sakurai, S Takasawa, H Okamoto, H Yamamoto
Affiliations
- PMID: 11457879
- PMCID: PMC203021
- DOI: 10.1172/JCI11771
Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice
Y Yamamoto et al. J Clin Invest. 2001 Jul.
Abstract
Vascular complications arising from multiple environmental and genetic factors are responsible for many of the disabilities and short life expectancy associated with diabetes mellitus. Here we provide the first direct in vivo evidence that interactions between advanced glycation end products (AGEs; nonenzymatically glycosylated protein derivatives formed during prolonged hyperglycemic exposure) and their receptor, RAGE, lead to diabetic vascular derangement. We created transgenic mice that overexpress human RAGE in vascular cells and crossbred them with another transgenic line that develops insulin-dependent diabetes shortly after birth. The resultant double transgenic mice exhibited increased hemoglobin A(1c) and serum AGE levels, as did the diabetic controls. The double transgenic mice demonstrated enlargement of the kidney, glomerular hypertrophy, increased albuminuria, mesangial expansion, advanced glomerulosclerosis, and increased serum creatinine compared with diabetic littermates lacking the RAGE transgene. To our knowledge, the development of this double transgenic mouse provides the first animal model that exhibits the renal changes seen in humans. Furthermore, the phenotypes of advanced diabetic nephropathy were prevented by administering an AGE inhibitor, (+/-)-2-isopropylidenehydrazono-4-oxo-thiazolidin-5-ylacetanilide (OPB-9195), thus establishing the AGE-RAGE system as a promising target for overcoming this aspect of diabetic pathogenesis.
Figures
Figure 1
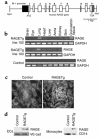
Generation and characterization of RAGE transgenic mice. (a) Transgene construct. The human RAGE genomic sequence, beginning with the initiator codon in exon 1 and ending 129 bp downstream from the last exon, was placed under the transcriptional control of the murine flk-1 promoter. The flk-1 fragment encompassed the 5′ untranslated region (dark gray box), sharing the ATG codon with the RAGE fragment. (b) Transgene-derived transcripts. Total RNAs isolated from various tissues of line 102 and 103 heterozygotes were analyzed by RT-PCR. The granulation tissue — the focus of angiogenesis — was prepared by punching out an area of the dorsal skin about 4–5 mm in diameter. The PCR-amplified products had a chain length of 354 bp as predicted and were sequence-verified. (c) Immunofluorescence staining of kidneys from line 102 RAGETg or nontransgenic control at 4 months of age using anti-human RAGE-specific polyclonal Ab. Original magnification, ×430. (d) Translation products of the transgene. Extracts of isolated ECs from renal cortex or peripheral blood monocytes of line 102 RAGETg or the nontransgenic control were immunodetected with the human RAGE Ab. Specific bands were marked at 55 kDa in line 102 RAGETg EC and monocyte extracts. VE-cad, VE-cadherin, i.e., an EC marker; CD14, a monocyte marker.
Figure 2
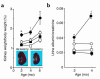
Renal changes in the early stage. (a) Kidney weight/body weight ratio. Inset: Sagittal section of the kidney at 4 months of age. Bar, 5 mm. (b) Albuminuria. Filled circles, DM+RAGETg+; open circles, DM+RAGETg–; filled squares, DM–RAGETg+; open squares, DM–RAGETg–. Data are mean ± SEM. *P < 0.02, †P < 0.05 compared with DM+RAGETg–. Statistical analysis was performed by t test.
Figure 3
Glomerular changes in the early stage. Glomerular cell proliferation (left) and glomerular volume (right) at 2 months of age. Data are mean ± SEM. *P < 0.05. Statistical analysis was performed by t test.
Figure 4
Renal changes in the late stage. PAS staining of thin kidney sections from line 102 DM+RAGETg+ (a), DM+RAGETg– (b), DM–RAGETg+ (c), and DM–RAGETg– (d) at 4 months of age and DM+RAGETg+ (e) at 8 months of age. Original magnification, ×670.
Figure 5
Mesangial expansion. Mesangium area (left) and mesangium fraction (right). Numbers on columns indicate months of age. Data are mean ± SEM. *P < 0.05. Statistical analysis was performed by t test.
Figure 6
(a) Sclerosis index and serum creatinine level. (b) Kidney weight/body weight ratio. Open bars, animals not treated with OPB-9195; filled columns, animals treated with OPB-9195. Numbers on columns indicate months of age. Data are mean ± SEM. *P < 0.05, †P < 0.005, #P < 0.001. Statistical analysis was performed by t test.
Figure 7
Stain for non-CML AGEs (a and b) and CML (c and d) in the kidneys from 4-month-old line 102 DM+RAGETg+ (a and c) and DM–RAGETg– (b and d) mice. Glomeruli were stained for non-CML AGEs and CML in DM+RAGETg– to extents similar to those in DM+RAGETg+ but were much less stained in DM–RAGETg+ and DM–RAGETg– mice. Original magnification, ×330. (e) Upregulation of mouse RAGE mRNA in diabetic glomeruli. Total RNAs isolated from glomeruli of each of the four groups in line 102 were analyzed by RT-PCR. mRAGE, mouse RAGE mRNA; hRAGE, human RAGE mRNA. Numbers indicate months of age.
Similar articles
- RAGE control of diabetic nephropathy in a mouse model: effects of RAGE gene disruption and administration of low-molecular weight heparin.
Myint KM, Yamamoto Y, Doi T, Kato I, Harashima A, Yonekura H, Watanabe T, Shinohara H, Takeuchi M, Tsuneyama K, Hashimoto N, Asano M, Takasawa S, Okamoto H, Yamamoto H. Myint KM, et al. Diabetes. 2006 Sep;55(9):2510-22. doi: 10.2337/db06-0221. Diabetes. 2006. PMID: 16936199 - Receptor for advanced glycation end products is a promising target of diabetic nephropathy.
Yamamoto Y, Doi T, Kato I, Shinohara H, Sakurai S, Yonekura H, Watanabe T, Myint KM, Harashima A, Takeuchi M, Takasawa S, Okamoto H, Hashimoto N, Asano M, Yamamoto H. Yamamoto Y, et al. Ann N Y Acad Sci. 2005 Jun;1043:562-6. doi: 10.1196/annals.1333.064. Ann N Y Acad Sci. 2005. PMID: 16037279 - An inhibitor of advanced glycation end product formation reduces N epsilon-(carboxymethyl)lysine accumulation in glomeruli of diabetic rats.
Nakamura S, Tachikawa T, Tobita K, Aoyama I, Takayama F, Enomoto A, Niwa T. Nakamura S, et al. Am J Kidney Dis. 2003 Mar;41(3 Suppl 1):S68-71. doi: 10.1053/ajkd.2003.50088. Am J Kidney Dis. 2003. PMID: 12612956 - Synergistic contributions of carbonyl stress and megsin in diabetic nephropathy.
Inagi R, Nangaku M, Miyata T. Inagi R, et al. Ann N Y Acad Sci. 2005 Jun;1043:605-8. doi: 10.1196/annals.1338.068. Ann N Y Acad Sci. 2005. PMID: 16037283 Review. - Can advanced glycation end product inhibitors modulate more than one pathway to enhance renoprotection in diabetes?
Coughlan MT, Cooper ME, Forbes JM. Coughlan MT, et al. Ann N Y Acad Sci. 2005 Jun;1043:750-8. doi: 10.1196/annals.1333.087. Ann N Y Acad Sci. 2005. PMID: 16037302 Review.
Cited by
- Bone fragility in type 2 diabetes mellitus.
Yamaguchi T. Yamaguchi T. World J Orthop. 2010 Nov 18;1(1):3-9. doi: 10.5312/wjo.v1.i1.3. World J Orthop. 2010. PMID: 22474621 Free PMC article. - Glycoxidation and diabetic complications: modern lessons and a warning?
Vlassara H, Uribarri J. Vlassara H, et al. Rev Endocr Metab Disord. 2004 Aug;5(3):181-8. doi: 10.1023/B:REMD.0000032406.84813.f6. Rev Endocr Metab Disord. 2004. PMID: 15211089 Review. No abstract available. - The RAGE/DIAPH1 Signaling Axis & Implications for the Pathogenesis of Diabetic Complications.
Ramasamy R, Shekhtman A, Schmidt AM. Ramasamy R, et al. Int J Mol Sci. 2022 Apr 21;23(9):4579. doi: 10.3390/ijms23094579. Int J Mol Sci. 2022. PMID: 35562970 Free PMC article. Review. - Markers of glycemic control in the mouse: comparisons of 6-h- and overnight-fasted blood glucoses to Hb A1c.
Han BG, Hao CM, Tchekneva EE, Wang YY, Lee CA, Ebrahim B, Harris RC, Kern TS, Wasserman DH, Breyer MD, Qi Z. Han BG, et al. Am J Physiol Endocrinol Metab. 2008 Oct;295(4):E981-6. doi: 10.1152/ajpendo.90283.2008. Epub 2008 Jul 29. Am J Physiol Endocrinol Metab. 2008. PMID: 18664598 Free PMC article. - Molecular mechanisms of diabetic vascular complications.
Kitada M, Zhang Z, Mima A, King GL. Kitada M, et al. J Diabetes Investig. 2010 Jun 1;1(3):77-89. doi: 10.1111/j.2040-1124.2010.00018.x. J Diabetes Investig. 2010. PMID: 24843412 Free PMC article. Review.
References
- Bojestig M, Arnqvist HJ, Hermansson G, Karlberg BE, Ludvigsson J. Declining incidence of nephropathy in insulin-dependent diabetes mellitus. N Engl J Med. 1994;330:15–18. - PubMed
- Krolewski M, Eggers PW, Warram JH. Magnitude of end-stage renal disease in IDDM: a 35 year follow-up study. Kidney Int. 1996;50:2041–2046. - PubMed
- Velasquez MT, Kimmel PL, Michaelis OE., IV Animal models of spontaneous diabetic kidney disease. FASEB J. 1990;4:2850–2859. - PubMed
- Doi T, et al. Glomerular lesions in nonobese diabetic mouse: before and after the onset of hyperglycemia. Lab Invest. 1990;63:204–212. - PubMed
- Williamson JR, et al. Increased vascular permeability in spontaneously diabetic BB/W rats and in rats with mild versus severe streptozotocin-induced diabetes. Prevention by aldose reductase inhibitors and castration. Diabetes. 1987;36:813–821. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases