Swift development of protective effector functions in naive CD8(+) T cells against malaria liver stages - PubMed (original) (raw)
Swift development of protective effector functions in naive CD8(+) T cells against malaria liver stages
G Sano et al. J Exp Med. 2001.
Abstract
We generated T cell receptor transgenic mice specific for the liver stages of the rodent malaria parasite Plasmodium yoelii and studied the early events in the development of in vivo effector functions in antigen-specific CD8(+) T cells. Differently to activated/memory cells, naive CD8(+) T cells are not capable of exerting antiparasitic activity unless previously primed by parasite immunization. While naive cells need to differentiate before achieving effector status, the time required for this process is very short. Indeed, interferon (IFN)-gamma and perforin mRNA are detectable 24 h after immunization and IFN-gamma secretion and cytotoxic activity are detected ex vivo 24 and 48 h after immunization, respectively. In contrast, the proliferation of CD8(+) T cells begins after 24 h and an increase in the total number of antigen-specific cells is detected only after 48 h. Remarkably, a strong CD8(+) T cell-mediated inhibition of parasite development is observed in mice challenged with viable parasites only 24 h after immunization with attenuated parasites. These results indicate that differentiation of naive CD8(+) T cells does not begin only after extensive cell division, rather this process precedes or occurs simultaneously with proliferation.
Figures
Figure 1
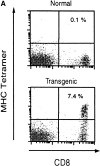
Phenotypic and functional specificity of the transgenic CD8+ T cells. (A) CD8+ T cells from transgenic CB6/F1 mice bind to SYVPSAEQI-tetramers. Plots were analyzed on live lymphocytes and the numbers indicated the frequency of CD8+ tetramer+ cells in total lymphocytes. (B) Adoptively transferred transgenic CD8+ T cells are activated only after immunization with radiation-attenuated P. yoelii sporozoites. The frequencies of SYVPSAEQI-specific IFN-γ–secreting cells in the spleen were determined by ELISPOT, 8 d after immunization, in the presence of SYVPSAEQI-coated target cells. Nonimmunized recipient mice and immunized normal mice were also used as controls. Results are expressed as average ± SD of duplicate cultures. Negligible numbers of spots are obtained using control target cells (data not shown).
Figure 1
Phenotypic and functional specificity of the transgenic CD8+ T cells. (A) CD8+ T cells from transgenic CB6/F1 mice bind to SYVPSAEQI-tetramers. Plots were analyzed on live lymphocytes and the numbers indicated the frequency of CD8+ tetramer+ cells in total lymphocytes. (B) Adoptively transferred transgenic CD8+ T cells are activated only after immunization with radiation-attenuated P. yoelii sporozoites. The frequencies of SYVPSAEQI-specific IFN-γ–secreting cells in the spleen were determined by ELISPOT, 8 d after immunization, in the presence of SYVPSAEQI-coated target cells. Nonimmunized recipient mice and immunized normal mice were also used as controls. Results are expressed as average ± SD of duplicate cultures. Negligible numbers of spots are obtained using control target cells (data not shown).
Figure 2
Activation kinetics of antigen-activated naive CD8+ T cells. (A) Spleen cells from immunized and nonimmunized recipient mice were isolated at 24, 48, and 72 h and stained for CD8 and with SYVPSAEQI-specific tetramers. Plots were analyzed on live lymphocytes and the numbers indicated represent the frequency of CD8+ tetramer+ T cells in total lymphocytes. (B) Spleen cells from immunized and nonimmunized recipient mice were analyzed by ELISPOT and tetramer staining on days 0, 2, 3, 4, 5, 6, 8, 10, 13, and 18 after immunization. Results are expressed as absolute number of SYVPSAEQI-specific CD8+ T cells per spleen calculated as the frequencies obtained by either ELISPOT (•, ○) or tetramer staining (▪, □ ) multiplied by the total number of cells obtained after spleen excision. Filled symbols represent data from immunized mice and open symbols represent data from nonimmunized mice.
Figure 2
Activation kinetics of antigen-activated naive CD8+ T cells. (A) Spleen cells from immunized and nonimmunized recipient mice were isolated at 24, 48, and 72 h and stained for CD8 and with SYVPSAEQI-specific tetramers. Plots were analyzed on live lymphocytes and the numbers indicated represent the frequency of CD8+ tetramer+ T cells in total lymphocytes. (B) Spleen cells from immunized and nonimmunized recipient mice were analyzed by ELISPOT and tetramer staining on days 0, 2, 3, 4, 5, 6, 8, 10, 13, and 18 after immunization. Results are expressed as absolute number of SYVPSAEQI-specific CD8+ T cells per spleen calculated as the frequencies obtained by either ELISPOT (•, ○) or tetramer staining (▪, □ ) multiplied by the total number of cells obtained after spleen excision. Filled symbols represent data from immunized mice and open symbols represent data from nonimmunized mice.
Figure 3
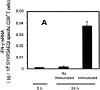
Rapid acquisition of effector functions and phenotypic activation in antigen-activated naive CD8+ T cells. (A and B) Tetramer+ spleen cells from immunized and nonimmunized recipient mice were isolated at 24 h and total mRNA was processed for RT-PCR to measure IFN-γ perforin and hypoxanthine ribosyltransferase (HPRT) as internal control (data not shown, and reference 15). The results are expressed as average ± SD of the amount of respective transcript per 106 tetramer+ cells. (C) Spleen and intrahepatic cells from immunized and nonimmunized (data not shown) recipient mice were isolated at 0, 24, and 48 h and analyzed by ELISPOT. Results are expressed as average ± SD of duplicate cultures. (D) 51Cr release assay using spleen cells from recipient mice obtained 48 h after immunization. Results are expressed as specific lysis at different target ratios of CD8+ T cells to SYVPSAEQI-coated target cells. Cells from immunized mice (▪, □) and nonimmunized mice (•, ○) were incubated 6 h with peptide-coated target cells (filled symbols) or target cells without peptide (open symbols). (E) Spleen cells from immunized and nonimmunized recipient mice stained for CD8 and CD44, and with SYVPSAEQI tetramers. Dark lines represent cells obtained at 0 h, while light lines represent cells obtained at 24 h. Histograms are gated on live CD8+ tetramer+ T cells.
Figure 3
Rapid acquisition of effector functions and phenotypic activation in antigen-activated naive CD8+ T cells. (A and B) Tetramer+ spleen cells from immunized and nonimmunized recipient mice were isolated at 24 h and total mRNA was processed for RT-PCR to measure IFN-γ perforin and hypoxanthine ribosyltransferase (HPRT) as internal control (data not shown, and reference 15). The results are expressed as average ± SD of the amount of respective transcript per 106 tetramer+ cells. (C) Spleen and intrahepatic cells from immunized and nonimmunized (data not shown) recipient mice were isolated at 0, 24, and 48 h and analyzed by ELISPOT. Results are expressed as average ± SD of duplicate cultures. (D) 51Cr release assay using spleen cells from recipient mice obtained 48 h after immunization. Results are expressed as specific lysis at different target ratios of CD8+ T cells to SYVPSAEQI-coated target cells. Cells from immunized mice (▪, □) and nonimmunized mice (•, ○) were incubated 6 h with peptide-coated target cells (filled symbols) or target cells without peptide (open symbols). (E) Spleen cells from immunized and nonimmunized recipient mice stained for CD8 and CD44, and with SYVPSAEQI tetramers. Dark lines represent cells obtained at 0 h, while light lines represent cells obtained at 24 h. Histograms are gated on live CD8+ tetramer+ T cells.
Figure 3
Rapid acquisition of effector functions and phenotypic activation in antigen-activated naive CD8+ T cells. (A and B) Tetramer+ spleen cells from immunized and nonimmunized recipient mice were isolated at 24 h and total mRNA was processed for RT-PCR to measure IFN-γ perforin and hypoxanthine ribosyltransferase (HPRT) as internal control (data not shown, and reference 15). The results are expressed as average ± SD of the amount of respective transcript per 106 tetramer+ cells. (C) Spleen and intrahepatic cells from immunized and nonimmunized (data not shown) recipient mice were isolated at 0, 24, and 48 h and analyzed by ELISPOT. Results are expressed as average ± SD of duplicate cultures. (D) 51Cr release assay using spleen cells from recipient mice obtained 48 h after immunization. Results are expressed as specific lysis at different target ratios of CD8+ T cells to SYVPSAEQI-coated target cells. Cells from immunized mice (▪, □) and nonimmunized mice (•, ○) were incubated 6 h with peptide-coated target cells (filled symbols) or target cells without peptide (open symbols). (E) Spleen cells from immunized and nonimmunized recipient mice stained for CD8 and CD44, and with SYVPSAEQI tetramers. Dark lines represent cells obtained at 0 h, while light lines represent cells obtained at 24 h. Histograms are gated on live CD8+ tetramer+ T cells.
Figure 3
Rapid acquisition of effector functions and phenotypic activation in antigen-activated naive CD8+ T cells. (A and B) Tetramer+ spleen cells from immunized and nonimmunized recipient mice were isolated at 24 h and total mRNA was processed for RT-PCR to measure IFN-γ perforin and hypoxanthine ribosyltransferase (HPRT) as internal control (data not shown, and reference 15). The results are expressed as average ± SD of the amount of respective transcript per 106 tetramer+ cells. (C) Spleen and intrahepatic cells from immunized and nonimmunized (data not shown) recipient mice were isolated at 0, 24, and 48 h and analyzed by ELISPOT. Results are expressed as average ± SD of duplicate cultures. (D) 51Cr release assay using spleen cells from recipient mice obtained 48 h after immunization. Results are expressed as specific lysis at different target ratios of CD8+ T cells to SYVPSAEQI-coated target cells. Cells from immunized mice (▪, □) and nonimmunized mice (•, ○) were incubated 6 h with peptide-coated target cells (filled symbols) or target cells without peptide (open symbols). (E) Spleen cells from immunized and nonimmunized recipient mice stained for CD8 and CD44, and with SYVPSAEQI tetramers. Dark lines represent cells obtained at 0 h, while light lines represent cells obtained at 24 h. Histograms are gated on live CD8+ tetramer+ T cells.
Figure 3
Rapid acquisition of effector functions and phenotypic activation in antigen-activated naive CD8+ T cells. (A and B) Tetramer+ spleen cells from immunized and nonimmunized recipient mice were isolated at 24 h and total mRNA was processed for RT-PCR to measure IFN-γ perforin and hypoxanthine ribosyltransferase (HPRT) as internal control (data not shown, and reference 15). The results are expressed as average ± SD of the amount of respective transcript per 106 tetramer+ cells. (C) Spleen and intrahepatic cells from immunized and nonimmunized (data not shown) recipient mice were isolated at 0, 24, and 48 h and analyzed by ELISPOT. Results are expressed as average ± SD of duplicate cultures. (D) 51Cr release assay using spleen cells from recipient mice obtained 48 h after immunization. Results are expressed as specific lysis at different target ratios of CD8+ T cells to SYVPSAEQI-coated target cells. Cells from immunized mice (▪, □) and nonimmunized mice (•, ○) were incubated 6 h with peptide-coated target cells (filled symbols) or target cells without peptide (open symbols). (E) Spleen cells from immunized and nonimmunized recipient mice stained for CD8 and CD44, and with SYVPSAEQI tetramers. Dark lines represent cells obtained at 0 h, while light lines represent cells obtained at 24 h. Histograms are gated on live CD8+ tetramer+ T cells.
Figure 4
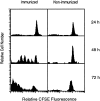
In vivo proliferation of antigen-activated naive CD8+ T cells. CFSE-labeled spleen cells from transgenic mice were transferred into normal mice and immunized with P. yoelii sporozoites. Spleen cells were isolated 24, 48, and 72 h after immunization and stained for CD8 and with SYVPSAEQI-tetramers. Spleen cells from nonimmunized mice were used as controls. Histograms are shown as CFSE dilution patterns gated on live lymphocytes and CD8+ tetramer+ T cells.
Figure 5
In vivo antiparasitic activity of CD8+ T cells. (A) Equal numbers of naive or activated/memory CD8+ tetramer+ T cells were transferred into normal mice and challenged with viable sporozoites. Activated/memory cells were obtained from recipient mice immunized with a recombinant vaccinia virus expressing the 9-mer SYVPSAEQI epitope at day 7. The parasite loads in the livers were measured by RT-PCR 40 h after challenge. The results are expressed as the average amount of parasite rRNA ± SD of three mice calculated based on competitor values. Numbers in parenthesis indicate the average percentage of inhibition compared with parasite load detected in normal mice. (B) The antiparasitic activity of activated CD8+ T cells is detectable in mice challenged 24 h after immunization. Mice receiving varying amounts of naive CD8+ tetramer+ T cells were immunized with attenuated sporozoites and 24 h later, challenged with viable sporozoites. The parasite loads in the livers were determined as described previously.
Figure 5
In vivo antiparasitic activity of CD8+ T cells. (A) Equal numbers of naive or activated/memory CD8+ tetramer+ T cells were transferred into normal mice and challenged with viable sporozoites. Activated/memory cells were obtained from recipient mice immunized with a recombinant vaccinia virus expressing the 9-mer SYVPSAEQI epitope at day 7. The parasite loads in the livers were measured by RT-PCR 40 h after challenge. The results are expressed as the average amount of parasite rRNA ± SD of three mice calculated based on competitor values. Numbers in parenthesis indicate the average percentage of inhibition compared with parasite load detected in normal mice. (B) The antiparasitic activity of activated CD8+ T cells is detectable in mice challenged 24 h after immunization. Mice receiving varying amounts of naive CD8+ tetramer+ T cells were immunized with attenuated sporozoites and 24 h later, challenged with viable sporozoites. The parasite loads in the livers were determined as described previously.
Similar articles
- Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites.
Cockburn IA, Chen YC, Overstreet MG, Lees JR, van Rooijen N, Farber DL, Zavala F. Cockburn IA, et al. PLoS Pathog. 2010 May 6;6(5):e1000877. doi: 10.1371/journal.ppat.1000877. PLoS Pathog. 2010. PMID: 20463809 Free PMC article. - Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes.
Trimnell A, Takagi A, Gupta M, Richie TL, Kappe SH, Wang R. Trimnell A, et al. J Immunol. 2009 Nov 1;183(9):5870-8. doi: 10.4049/jimmunol.0900302. Epub 2009 Oct 7. J Immunol. 2009. PMID: 19812194 - The complexity of protective immunity against liver-stage malaria.
Doolan DL, Hoffman SL. Doolan DL, et al. J Immunol. 2000 Aug 1;165(3):1453-62. doi: 10.4049/jimmunol.165.3.1453. J Immunol. 2000. PMID: 10903750 - Effector and memory CD8+ T cells as seen in immunity to malaria.
Morrot A, Zavala F. Morrot A, et al. Immunol Rev. 2004 Oct;201:291-303. doi: 10.1111/j.0105-2896.2004.00175.x. Immunol Rev. 2004. PMID: 15361248 Review. - Regulation of the CD8+ T cell responses against Plasmodium liver stages in mice.
Morrot A, Zavala F. Morrot A, et al. Int J Parasitol. 2004 Dec;34(13-14):1529-34. doi: 10.1016/j.ijpara.2004.10.001. Int J Parasitol. 2004. PMID: 15582529 Review.
Cited by
- A sufficient role of MHC class I molecules on hepatocytes in anti-plasmodial activity of CD8 (+) T cells in vivo.
Huang J, Tsao T, Zhang M, Rai U, Tsuji M, Li X. Huang J, et al. Front Microbiol. 2015 Feb 12;6:69. doi: 10.3389/fmicb.2015.00069. eCollection 2015. Front Microbiol. 2015. PMID: 25729379 Free PMC article. - Immune responses to malaria pre-erythrocytic stages: Implications for vaccine development.
Abuga KM, Jones-Warner W, Hafalla JCR. Abuga KM, et al. Parasite Immunol. 2021 Feb;43(2):e12795. doi: 10.1111/pim.12795. Epub 2020 Oct 9. Parasite Immunol. 2021. PMID: 32981095 Free PMC article. Review. - The onset of CD8+-T-cell contraction is influenced by the peak of Listeria monocytogenes infection and antigen display.
Porter BB, Harty JT. Porter BB, et al. Infect Immun. 2006 Mar;74(3):1528-36. doi: 10.1128/IAI.74.3.1528-1536.2006. Infect Immun. 2006. PMID: 16495523 Free PMC article. - Modulation of CD4(+) T cell-dependent specific cytotoxic CD8(+) T cells differentiation and proliferation by the timing of increase in the pathogen load.
Tzelepis F, Persechini PM, Rodrigues MM. Tzelepis F, et al. PLoS One. 2007 Apr 25;2(4):e393. doi: 10.1371/journal.pone.0000393. PLoS One. 2007. PMID: 17460760 Free PMC article. - Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites.
Cockburn IA, Chen YC, Overstreet MG, Lees JR, van Rooijen N, Farber DL, Zavala F. Cockburn IA, et al. PLoS Pathog. 2010 May 6;6(5):e1000877. doi: 10.1371/journal.ppat.1000877. PLoS Pathog. 2010. PMID: 20463809 Free PMC article.
References
- Veiga-Fernandes H., Walter U., Bourgeois C., McLean A., Rocha B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo . Nat. Immunol. 2000;1:47–53. - PubMed
- Iezzi G., Karjalainen K., Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. - PubMed
- Romero P., Maryanski J.L., Corradin G., Nussenzweig R.S., Nussenzweig V., Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–326. - PubMed
- Rodrigues M.M., Cordey A.S., Arreaza G., Corradin G., Romero P., Maryanski J.L., Nussenzweig R.S., Zavala F. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 1991;3:579–585. - PubMed
- Rodrigues M., Li S., Murata K., Rodriguez D., Rodriguez J.R., Bacik I., Bennink J.R., Yewdell J.W., Garcia-Sastre A., Nussenzweig R.S. Influenza and vaccinia viruses expressing malaria CD8+ T and B cell epitopes. Comparison of their immunogenicity and capacity to induce protective immunity. J. Immunol. 1994;153:4636–4648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials