Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection - PubMed (original) (raw)
Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection
C M Collazo et al. J Exp Med. 2001.
Abstract
The cytokine interferon (IFN)-gamma regulates immune clearance of parasitic, bacterial, and viral infections; however, the underlying mechanisms are poorly understood. Recently, a family of IFN-gamma-induced genes has been identified that encode 48-kD GTP-binding proteins that localize to the endoplasmic reticulum of cells. The prototype of this family, IGTP, has been shown to be required for host defense against acute infections with the protozoan parasite Toxoplasma gondii, but not for normal clearance of the bacterium Listeria monocytogenes and murine cytomegalovirus (MCMV). To determine whether other members of the gene family also play important roles in immune defense, we generated mice that lacked expression of the genes LRG-47 and IRG-47, and examined their responses to representative pathogens. After infection with T. gondii, LRG-47-deficient mice succumbed uniformly and rapidly during the acute phase of the infection; in contrast, IRG-47-deficient mice displayed only partially decreased resistance that was not manifested until the chronic phase. After infection with L. monocytogenes, LRG-47-deficient mice exhibited a profound loss of resistance, whereas IRG-47-deficient mice exhibited completely normal resistance. In addition, both strains displayed normal clearance of MCMV. Thus, LRG-47 and IRG-47 have vital, but distinct roles in immune defense against protozoan and bacterial infections.
Figures
Figure 1
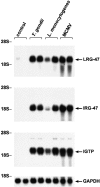
Induction of LRG-47 and IRG-47 expression in response to different pathogens. Pairs of mice were inoculated as indicated with 20 cysts T. gondii for 8 d, 1,000 CFU L. monocytogenes for 5 d, or 5 × 104 PFU MCMV for 36 h, or were left uninfected (control). Total RNA was prepared from liver and used for sequential Northern blotting with LRG-47, IRG-47, IGTP, and GAPDH probes. Positions of the major ribosomal RNA species are indicated.
Figure 3
Acute loss of resistance to T. gondii in LRG-47–deficient mice. The indicated strains were inoculated intraperitoneally with 20 cysts T. gondii, and their ability to restrict the infection was assessed. (A) Wild-type (n = 6) and LRG-47–deficient mice (n = 6) were monitored for their survival for 40 d. KO, knockout. (B) Peritoneal exudate cells from wild-type (WT; n = 3), IFN-γ–deficient (n = 3), and LRG-47–deficient mice (n = 4) were isolated at 5 d after infection, and the presence of intracellular T. gondii was determined microscopically. (C) Sera were isolated from wild-type (n = 2) and LRG-47–deficient mice (n = 4) at 5 d after infection and used for IFN-γ and IL-12 determination by ELISA. A–C are representative results of two experiments.
Figure 3
Acute loss of resistance to T. gondii in LRG-47–deficient mice. The indicated strains were inoculated intraperitoneally with 20 cysts T. gondii, and their ability to restrict the infection was assessed. (A) Wild-type (n = 6) and LRG-47–deficient mice (n = 6) were monitored for their survival for 40 d. KO, knockout. (B) Peritoneal exudate cells from wild-type (WT; n = 3), IFN-γ–deficient (n = 3), and LRG-47–deficient mice (n = 4) were isolated at 5 d after infection, and the presence of intracellular T. gondii was determined microscopically. (C) Sera were isolated from wild-type (n = 2) and LRG-47–deficient mice (n = 4) at 5 d after infection and used for IFN-γ and IL-12 determination by ELISA. A–C are representative results of two experiments.
Figure 3
Acute loss of resistance to T. gondii in LRG-47–deficient mice. The indicated strains were inoculated intraperitoneally with 20 cysts T. gondii, and their ability to restrict the infection was assessed. (A) Wild-type (n = 6) and LRG-47–deficient mice (n = 6) were monitored for their survival for 40 d. KO, knockout. (B) Peritoneal exudate cells from wild-type (WT; n = 3), IFN-γ–deficient (n = 3), and LRG-47–deficient mice (n = 4) were isolated at 5 d after infection, and the presence of intracellular T. gondii was determined microscopically. (C) Sera were isolated from wild-type (n = 2) and LRG-47–deficient mice (n = 4) at 5 d after infection and used for IFN-γ and IL-12 determination by ELISA. A–C are representative results of two experiments.
Figure 2
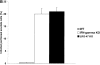
Gene targeting to create LRG-47– and IRG-47–deficient mice. As described in detail in the Materials and Methods, standard gene targeting techniques were used to generate mice that lack production of LRG-47 and IRG-47. Western blotting was then used to verify absence of protein expression. (A) Embryonic fibroblasts from wild-type (+/+) or LRG-47–deficient (−/−) mice were exposed to control conditions or 100 U/ml IFN-γ for 15 h. Lysates were prepared from the cells, resolved by 10% SDS-PAGE, and used for sequential Western blotting with anti–LRG-47 and anti-IGTP antisera. (B) Spleen and thymus were isolated from wild-type (+/+) or IRG-47–deficient (−/−) mice. Lysates were prepared, resolved by 10% SDS-PAGE, and used for Western blotting with anti–IRG-47 antisera. Expression in IRG-47–deficient fibroblasts could not be assessed because of cross-reacting bands in the fibroblast lysates that were recognized by the anti–IRG-47 antisera.
Figure 2
Gene targeting to create LRG-47– and IRG-47–deficient mice. As described in detail in the Materials and Methods, standard gene targeting techniques were used to generate mice that lack production of LRG-47 and IRG-47. Western blotting was then used to verify absence of protein expression. (A) Embryonic fibroblasts from wild-type (+/+) or LRG-47–deficient (−/−) mice were exposed to control conditions or 100 U/ml IFN-γ for 15 h. Lysates were prepared from the cells, resolved by 10% SDS-PAGE, and used for sequential Western blotting with anti–LRG-47 and anti-IGTP antisera. (B) Spleen and thymus were isolated from wild-type (+/+) or IRG-47–deficient (−/−) mice. Lysates were prepared, resolved by 10% SDS-PAGE, and used for Western blotting with anti–IRG-47 antisera. Expression in IRG-47–deficient fibroblasts could not be assessed because of cross-reacting bands in the fibroblast lysates that were recognized by the anti–IRG-47 antisera.
Figure 5
Marked loss of resistance to L. monocytogenes in LRG-47–deficient mice. The indicated strains were inoculated intraperitoneally with 1,000 CFU L. monocytogenes, and their ability to restrict the infection was assessed. (A) Wild-type (n = 4) and LRG-47–deficient mice (n = 6) were monitored for their survival for 40 d. KO, knockout. (B) Spleen and liver of wild-type (WT; n = 7), IFN-γ–deficient (n = 7), and LRG-47–deficient mice (n = 9) were isolated at 3 d after infection, and the numbers of bacteria present was determined. Statistical analysis for wild-type vs. LRG-47–deficient mice: spleen, P = 0.003; liver, P = 0.05. (C) Sera were isolated from wild-type (n = 3) and LRG-47–deficient mice (n = 5) at 3 d after infection and used for IFN-γ and IL-12 determination by ELISA. A–C are representative results from two experiments.
Figure 5
Marked loss of resistance to L. monocytogenes in LRG-47–deficient mice. The indicated strains were inoculated intraperitoneally with 1,000 CFU L. monocytogenes, and their ability to restrict the infection was assessed. (A) Wild-type (n = 4) and LRG-47–deficient mice (n = 6) were monitored for their survival for 40 d. KO, knockout. (B) Spleen and liver of wild-type (WT; n = 7), IFN-γ–deficient (n = 7), and LRG-47–deficient mice (n = 9) were isolated at 3 d after infection, and the numbers of bacteria present was determined. Statistical analysis for wild-type vs. LRG-47–deficient mice: spleen, P = 0.003; liver, P = 0.05. (C) Sera were isolated from wild-type (n = 3) and LRG-47–deficient mice (n = 5) at 3 d after infection and used for IFN-γ and IL-12 determination by ELISA. A–C are representative results from two experiments.
Figure 5
Marked loss of resistance to L. monocytogenes in LRG-47–deficient mice. The indicated strains were inoculated intraperitoneally with 1,000 CFU L. monocytogenes, and their ability to restrict the infection was assessed. (A) Wild-type (n = 4) and LRG-47–deficient mice (n = 6) were monitored for their survival for 40 d. KO, knockout. (B) Spleen and liver of wild-type (WT; n = 7), IFN-γ–deficient (n = 7), and LRG-47–deficient mice (n = 9) were isolated at 3 d after infection, and the numbers of bacteria present was determined. Statistical analysis for wild-type vs. LRG-47–deficient mice: spleen, P = 0.003; liver, P = 0.05. (C) Sera were isolated from wild-type (n = 3) and LRG-47–deficient mice (n = 5) at 3 d after infection and used for IFN-γ and IL-12 determination by ELISA. A–C are representative results from two experiments.
Figure 4
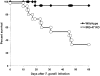
Marginal loss of resistance to T. gondii in IRG-47–deficient mice. Wild-type (n = 17) and IRG-47–deficient mice (n = 15) were monitored for their survival for 60 d. Shown are the cumulative results of two experiments. KO, knockout.
Figure 6
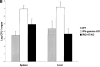
Normal resistance to L. monocytogenes in IRG-47–deficient mice. (A) Wild-type (WT; n = 6), IFN-γ–deficient (n = 6), and IRG-47–deficient mice (n = 6) were monitored for their survival for 40 d. KO, knockout. (B) Spleen and liver of wild-type (n = 4), IFN-γ–deficient (n = 3), and IRG-47–deficient mice (n = 5) were isolated at 3 d after infection, and the number of bacteria present was determined. Statistical analysis for wild-type vs. IRG-47–deficient mice: spleen, P = 0.01; liver, P = 0.28. The slight increase in the splenic bacterial loads of IRG-47–deficient mice, compared with that of wild-type mice, was not seen in a second experiment. A and B are representative results from two experiments.
Figure 6
Normal resistance to L. monocytogenes in IRG-47–deficient mice. (A) Wild-type (WT; n = 6), IFN-γ–deficient (n = 6), and IRG-47–deficient mice (n = 6) were monitored for their survival for 40 d. KO, knockout. (B) Spleen and liver of wild-type (n = 4), IFN-γ–deficient (n = 3), and IRG-47–deficient mice (n = 5) were isolated at 3 d after infection, and the number of bacteria present was determined. Statistical analysis for wild-type vs. IRG-47–deficient mice: spleen, P = 0.01; liver, P = 0.28. The slight increase in the splenic bacterial loads of IRG-47–deficient mice, compared with that of wild-type mice, was not seen in a second experiment. A and B are representative results from two experiments.
Similar articles
- Pathogen-specific loss of host resistance in mice lacking the IFN-gamma-inducible gene IGTP.
Taylor GA, Collazo CM, Yap GS, Nguyen K, Gregorio TA, Taylor LS, Eagleson B, Secrest L, Southon EA, Reid SW, Tessarollo L, Bray M, McVicar DW, Komschlies KL, Young HA, Biron CA, Sher A, Vande Woude GF. Taylor GA, et al. Proc Natl Acad Sci U S A. 2000 Jan 18;97(2):751-5. doi: 10.1073/pnas.97.2.751. Proc Natl Acad Sci U S A. 2000. PMID: 10639151 Free PMC article. - The IFN-γ-inducible GTPase, Irga6, protects mice against Toxoplasma gondii but not against Plasmodium berghei and some other intracellular pathogens.
Liesenfeld O, Parvanova I, Zerrahn J, Han SJ, Heinrich F, Muñoz M, Kaiser F, Aebischer T, Buch T, Waisman A, Reichmann G, Utermöhlen O, von Stebut E, von Loewenich FD, Bogdan C, Specht S, Saeftel M, Hoerauf A, Mota MM, Könen-Waisman S, Kaufmann SH, Howard JC. Liesenfeld O, et al. PLoS One. 2011;6(6):e20568. doi: 10.1371/journal.pone.0020568. Epub 2011 Jun 17. PLoS One. 2011. PMID: 21698150 Free PMC article. - p47 GTPases regulate Toxoplasma gondii survival in activated macrophages.
Butcher BA, Greene RI, Henry SC, Annecharico KL, Weinberg JB, Denkers EY, Sher A, Taylor GA. Butcher BA, et al. Infect Immun. 2005 Jun;73(6):3278-86. doi: 10.1128/IAI.73.6.3278-3286.2005. Infect Immun. 2005. PMID: 15908352 Free PMC article. - Toxoplasma gondii and the Immunity-Related GTPase (IRG) resistance system in mice: a review.
Zhao YO, Rohde C, Lilue JT, Könen-Waisman S, Khaminets A, Hunn JP, Howard JC. Zhao YO, et al. Mem Inst Oswaldo Cruz. 2009 Mar;104(2):234-40. doi: 10.1590/s0074-02762009000200016. Mem Inst Oswaldo Cruz. 2009. PMID: 19430648 Review. - Decoding Toxoplasma gondii virulence: the mechanisms of IRG protein inactivation.
Murillo-Léon M, Bastidas-Quintero AM, Steinfeldt T. Murillo-Léon M, et al. Trends Parasitol. 2024 Sep;40(9):805-819. doi: 10.1016/j.pt.2024.07.009. Epub 2024 Aug 20. Trends Parasitol. 2024. PMID: 39168720 Review.
Cited by
- The GTPase activity of murine guanylate-binding protein 2 (mGBP2) controls the intracellular localization and recruitment to the parasitophorous vacuole of Toxoplasma gondii.
Kravets E, Degrandi D, Weidtkamp-Peters S, Ries B, Konermann C, Felekyan S, Dargazanli JM, Praefcke GJ, Seidel CA, Schmitt L, Smits SH, Pfeffer K. Kravets E, et al. J Biol Chem. 2012 Aug 10;287(33):27452-66. doi: 10.1074/jbc.M112.379636. Epub 2012 Jun 22. J Biol Chem. 2012. PMID: 22730319 Free PMC article. - Mycobacterium tuberculosis Phosphate Uptake System Component PstA2 Is Not Required for Gene Regulation or Virulence.
Tischler AD, Leistikow RL, Ramakrishnan P, Voskuil MI, McKinney JD. Tischler AD, et al. PLoS One. 2016 Aug 24;11(8):e0161467. doi: 10.1371/journal.pone.0161467. eCollection 2016. PLoS One. 2016. PMID: 27557082 Free PMC article. - Mammalian Atg8 proteins and the autophagy factor IRGM control mTOR and TFEB at a regulatory node critical for responses to pathogens.
Kumar S, Jain A, Choi SW, da Silva GPD, Allers L, Mudd MH, Peters RS, Anonsen JH, Rusten TE, Lazarou M, Deretic V. Kumar S, et al. Nat Cell Biol. 2020 Aug;22(8):973-985. doi: 10.1038/s41556-020-0549-1. Epub 2020 Aug 3. Nat Cell Biol. 2020. PMID: 32753672 Free PMC article. - Regulation of hippocampal neuronal apoptosis and autophagy in mice with sepsis-associated encephalopathy by immunity-related GTPase M1.
Zhou RX, Li YY, Qu Y, Huang Q, Sun XM, Mu DZ, Li XH. Zhou RX, et al. CNS Neurosci Ther. 2020 Feb;26(2):177-188. doi: 10.1111/cns.13229. Epub 2019 Oct 14. CNS Neurosci Ther. 2020. PMID: 31612615 Free PMC article. - Immunity-related GTPase IRGM at the intersection of autophagy, inflammation, and tumorigenesis.
Goswami AB, Karadarević D, Castaño-Rodríguez N. Goswami AB, et al. Inflamm Res. 2022 Aug;71(7-8):785-795. doi: 10.1007/s00011-022-01595-x. Epub 2022 Jun 14. Inflamm Res. 2022. PMID: 35699756 Free PMC article. Review.
References
- Stark G.R., Kerr I.M., Williams B.R.G., Silverman R.H., Shreiber R.D. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. - PubMed
- Boehm U., Klamp T., Groot M., Howard J.C. Cellular responses to interferon-γ. Annu. Rev. Immunol. 1997;15:749–795. - PubMed
- Sorace J.M., Johnson R.J., Howard D.L., Drysdale B.E. Identification of an endotoxin and IFN-γ-inducible cDNApossible identification of a novel protein family. J. Leukoc. Biol. 1995;58:477–484. - PubMed
- Taylor G.A., Stauber R., Rulong S., Hudson S.E., Pei V., Pavlakis G.N., Resau J.H., Vande Woude G.F. The inducibly expressed GTPase (IGTP) localizes to the endoplasmic reticulum independently of GTP binding. J. Biol. Chem. 1997;272:10639–10645. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous