Regulation of myostatin activity and muscle growth - PubMed (original) (raw)
Regulation of myostatin activity and muscle growth
S J Lee et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Myostatin is a transforming growth factor-beta family member that acts as a negative regulator of skeletal muscle mass. To identify possible myostatin inhibitors that may have applications for promoting muscle growth, we investigated the regulation of myostatin signaling. Myostatin protein purified from mammalian cells consisted of a noncovalently held complex of the N-terminal propeptide and a disulfide-linked dimer of C-terminal fragments. The purified C-terminal myostatin dimer was capable of binding the activin type II receptors, Act RIIB and, to a lesser extent, Act RIIA. Binding of myostatin to Act RIIB could be inhibited by the activin-binding protein follistatin and, at higher concentrations, by the myostatin propeptide. To determine the functional significance of these interactions in vivo, we generated transgenic mice expressing high levels of the propeptide, follistatin, or a dominant-negative form of Act RIIB by using a skeletal muscle-specific promoter. Independent transgenic mouse lines for each construct exhibited dramatic increases in muscle mass comparable to those seen in myostatin knockout mice. Our findings suggest that the propeptide, follistatin, or other molecules that block signaling through this pathway may be useful agents for enhancing muscle growth for both human therapeutic and agricultural applications.
Figures
Figure 1
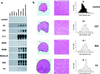
Binding of myostatin to activin type II receptors. (a) Analysis of purified myostatin protein. Myostatin protein preparation following the heparin column (heparin eluate) or following reverse-phase HPLC (fractions 32–34 or 35–37 containing the C-terminal region or propeptide, respectively) was electrophoresed under reducing (+βME) or nonreducing (−βME) conditions and either silver stained or subjected to Western analysis. (b) Binding of myostatin to lentil lectin. The heparin eluate was bound to lentil lectin Sepharose and eluted with methyl mannose. Samples were electrophoresed under reducing conditions, blotted, and probed with antibodies directed against the C-terminal region. Similar analysis by using antibodies directed against the pro region showed that the pro region was also retained on the column and eluted with methyl mannose (data not shown). (c) Crosslinking experiments. COS-7 cells transfected with expression constructs for the indicated receptors were incubated with 125I-myostatin followed by the crosslinking agent disuccinimidyl suberate. Crosslinked complexes were analyzed by SDS/PAGE. Asterisk denotes predicted size for myostatin bound to an activin type II receptor. In the rightmost lane, excess unlabeled myostatin was included in the binding reaction. (d) Binding of myostatin to ActRIIB. All points represent the average of triplicate samples. (e) Scatchard analysis of the data shown in d. (f) Inhibition of myostatin binding to ActRIIB by follistatin (diamonds) and the propeptide (circles). Each experiment was carried out in triplicate, and each curve represents the average of three independent experiments.
Figure 2
Increased muscling in mice overexpressing a dominant-negative form of ActRIIB or full length follistatin. A control male nontransgenic mouse, a male transgenic mouse from the C11 line (dominant-negative ActRIIB), and the F3 male founder mouse (follistatin) are shown. Pictures of live mice are shown in the top row, and pictures of animals that had been killed and skinned are shown in the bottom three rows.
Figure 3
Analysis of transgenic mice. (a) Specific expression of transgenes in skeletal muscle. Northern analysis of RNA samples prepared from female mice was carried out by using simian virus 40 sequences as a probe. On longer exposures of these blots, no expression of the transgenes was observed in liver, kidney, spleen, or heart in any of these lines. (b) Muscle analysis. Sections were stained with hematoxylin and eosin. The gastrocnemius and plantaris muscles are outlined (Left). Center shows higher magnifications of representative areas from the gastrocnemius muscle. Right shows distribution of fiber diameters. Each graph represents the composite of 450 fiber measurements from 3 animals (150 per animal), except for the F3 graph, which represents 175 measurements from the F3 founder animal. Standard deviations of fiber sizes were 9, 11, 11, and 13 μm for control, B32, C27, and F3 animals, respectively. Black and gray arrows show the mean fiber diameters for control and transgenic animals, respectively. Note that in each case, the mean fiber diameter was increased in the transgenic animals (P < 0.001).
Similar articles
- Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases.
Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ. Wolfman NM, et al. Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15842-6. doi: 10.1073/pnas.2534946100. Epub 2003 Dec 11. Proc Natl Acad Sci U S A. 2003. PMID: 14671324 Free PMC article. - Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice.
Nakatani M, Takehara Y, Sugino H, Matsumoto M, Hashimoto O, Hasegawa Y, Murakami T, Uezumi A, Takeda S, Noji S, Sunada Y, Tsuchida K. Nakatani M, et al. FASEB J. 2008 Feb;22(2):477-87. doi: 10.1096/fj.07-8673com. Epub 2007 Sep 24. FASEB J. 2008. PMID: 17893249 - Regulation of muscle growth by multiple ligands signaling through activin type II receptors.
Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, Wright JF, Barker C, Ehrmantraut G, Holmstrom J, Trowell B, Gertz B, Jiang MS, Sebald SM, Matzuk M, Li E, Liang LF, Quattlebaum E, Stotish RL, Wolfman NM. Lee SJ, et al. Proc Natl Acad Sci U S A. 2005 Dec 13;102(50):18117-22. doi: 10.1073/pnas.0505996102. Epub 2005 Dec 5. Proc Natl Acad Sci U S A. 2005. PMID: 16330774 Free PMC article. - Role of myostatin in metabolism.
Gonzalez-Cadavid NF, Bhasin S. Gonzalez-Cadavid NF, et al. Curr Opin Clin Nutr Metab Care. 2004 Jul;7(4):451-7. doi: 10.1097/01.mco.0000134365.99523.7f. Curr Opin Clin Nutr Metab Care. 2004. PMID: 15192449 Review. - Transforming growth factor-beta and myostatin signaling in skeletal muscle.
Kollias HD, McDermott JC. Kollias HD, et al. J Appl Physiol (1985). 2008 Mar;104(3):579-87. doi: 10.1152/japplphysiol.01091.2007. Epub 2007 Nov 21. J Appl Physiol (1985). 2008. PMID: 18032576 Review.
Cited by
- Embryonic transcriptome unravels mechanisms and pathways underlying embryonic development with respect to muscle growth, egg production, and plumage formation in native and broiler chickens.
Kanakachari M, Ashwini R, Chatterjee RN, Bhattacharya TK. Kanakachari M, et al. Front Genet. 2022 Oct 14;13:990849. doi: 10.3389/fgene.2022.990849. eCollection 2022. Front Genet. 2022. PMID: 36313432 Free PMC article. - Preclinical insights into the gut-skeletal muscle axis in chronic gastrointestinal diseases.
Ehlers L, Bannert K, Rohde S, Berlin P, Reiner J, Wiese M, Doller J, Lerch MM, Aghdassi AA, Meyer F, Valentini L, Agrifoglio O, Metges CC, Lamprecht G, Jaster R. Ehlers L, et al. J Cell Mol Med. 2020 Aug;24(15):8304-8314. doi: 10.1111/jcmm.15554. Epub 2020 Jul 6. J Cell Mol Med. 2020. PMID: 32628812 Free PMC article. Review. - TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology.
Morikawa M, Derynck R, Miyazono K. Morikawa M, et al. Cold Spring Harb Perspect Biol. 2016 May 2;8(5):a021873. doi: 10.1101/cshperspect.a021873. Cold Spring Harb Perspect Biol. 2016. PMID: 27141051 Free PMC article. Review. - Antimyostatin Treatment in Health and Disease: The Story of Great Expectations and Limited Success.
Nielsen TL, Vissing J, Krag TO. Nielsen TL, et al. Cells. 2021 Mar 3;10(3):533. doi: 10.3390/cells10030533. Cells. 2021. PMID: 33802348 Free PMC article. Review. - Sex-specific increases in myostatin and SMAD3 contribute to obesity-related insulin resistance in human skeletal muscle and primary human myotubes.
Saxena G, Gallagher S, Law TD, Maschari D, Walsh E, Dudley C, Brault JJ, Consitt LA. Saxena G, et al. Am J Physiol Endocrinol Metab. 2024 Mar 1;326(3):E352-E365. doi: 10.1152/ajpendo.00199.2023. Epub 2023 Dec 13. Am J Physiol Endocrinol Metab. 2024. PMID: 38088865
References
- McPherron A C, Lawler A M, Lee S-J. Nature (London) 1997;387:83–90. - PubMed
- Grobet L, Martin L J R, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Ménissier F, Massabanda J, et al. Nat Genet. 1997;17:71–74. - PubMed
- Kambadur R, Sharma M, Smith T P L, Bass J J. Genome Res. 1997;7:910–916. - PubMed
- Grobet L, Poncelet D, Royo L J, Brouwers B, Pirottin D, Michaux C, Ménissier F, Zanotti M, Dunner S, Georges M. Mamm Genome. 1998;9:210–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous