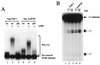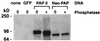Identification and functional characterization of neo-poly(A) polymerase, an RNA processing enzyme overexpressed in human tumors - PubMed (original) (raw)
Identification and functional characterization of neo-poly(A) polymerase, an RNA processing enzyme overexpressed in human tumors
S L Topalian et al. Mol Cell Biol. 2001 Aug.
Abstract
Poly(A) polymerase (PAP) plays an essential role in polyadenylation of mRNA precursors, and it has long been thought that mammalian cells contain only a single PAP gene. We describe here the unexpected existence of a human PAP, which we call neo-PAP, encoded by a previously uncharacterized gene. cDNA was isolated from a tumor-derived cDNA library encoding an 82.8-kDa protein bearing 71% overall similarity to human PAP. Strikingly, the organization of the two PAP genes is nearly identical, indicating that they arose from a common ancestor. Neo-PAP and PAP were indistinguishable in in vitro assays of both specific and nonspecific polyadenylation and also endonucleolytic cleavage. Neo-PAP produced by transfection was exclusively nuclear, as demonstrated by immunofluorescence microscopy. However, notable sequence divergence between the C-terminal domains of neo-PAP and PAP suggested that the two enzymes might be differentially regulated. While PAP is phosphorylated throughout the cell cycle and hyperphosphorylated during M phase, neo-PAP did not show evidence of phosphorylation on Western blot analysis, which was unexpected in the context of a conserved cyclin recognition motif and multiple potential cyclin-dependent kinase (cdk) phosphorylation sites. Intriguingly, Northern blot analysis demonstrated that each PAP displayed distinct mRNA splice variants, and both PAP mRNAs were significantly overexpressed in human cancer cells compared to expression in normal or virally transformed cells. Neo-PAP may therefore be an important RNA processing enzyme that is regulated by a mechanism distinct from that utilized by PAP.
Figures
FIG. 1
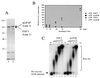
Nucleotide and amino acid sequences of neo-PAP. (A) cDNA and translated protein sequences of neo-PAP, with initiation and termination codons bolded and underlined. (B) Similarity between neo-PAP and human PAP II protein sequences. (C) Alignment of amino acid sequences of the C-terminal domains of neo-PAP and human PAP II. Asterisks indicate NLSs, nonconsensus cdk sites are in bold, consensus cdk sites are in bold and are underlined, plus signs indicate conserved amino acids, and minus signs indicate that no amino acid is present.
FIG. 1
Nucleotide and amino acid sequences of neo-PAP. (A) cDNA and translated protein sequences of neo-PAP, with initiation and termination codons bolded and underlined. (B) Similarity between neo-PAP and human PAP II protein sequences. (C) Alignment of amino acid sequences of the C-terminal domains of neo-PAP and human PAP II. Asterisks indicate NLSs, nonconsensus cdk sites are in bold, consensus cdk sites are in bold and are underlined, plus signs indicate conserved amino acids, and minus signs indicate that no amino acid is present.
FIG. 1
Nucleotide and amino acid sequences of neo-PAP. (A) cDNA and translated protein sequences of neo-PAP, with initiation and termination codons bolded and underlined. (B) Similarity between neo-PAP and human PAP II protein sequences. (C) Alignment of amino acid sequences of the C-terminal domains of neo-PAP and human PAP II. Asterisks indicate NLSs, nonconsensus cdk sites are in bold, consensus cdk sites are in bold and are underlined, plus signs indicate conserved amino acids, and minus signs indicate that no amino acid is present.
FIG. 1
Nucleotide and amino acid sequences of neo-PAP. (A) cDNA and translated protein sequences of neo-PAP, with initiation and termination codons bolded and underlined. (B) Similarity between neo-PAP and human PAP II protein sequences. (C) Alignment of amino acid sequences of the C-terminal domains of neo-PAP and human PAP II. Asterisks indicate NLSs, nonconsensus cdk sites are in bold, consensus cdk sites are in bold and are underlined, plus signs indicate conserved amino acids, and minus signs indicate that no amino acid is present.
FIG. 1
Nucleotide and amino acid sequences of neo-PAP. (A) cDNA and translated protein sequences of neo-PAP, with initiation and termination codons bolded and underlined. (B) Similarity between neo-PAP and human PAP II protein sequences. (C) Alignment of amino acid sequences of the C-terminal domains of neo-PAP and human PAP II. Asterisks indicate NLSs, nonconsensus cdk sites are in bold, consensus cdk sites are in bold and are underlined, plus signs indicate conserved amino acids, and minus signs indicate that no amino acid is present.
FIG. 1
Nucleotide and amino acid sequences of neo-PAP. (A) cDNA and translated protein sequences of neo-PAP, with initiation and termination codons bolded and underlined. (B) Similarity between neo-PAP and human PAP II protein sequences. (C) Alignment of amino acid sequences of the C-terminal domains of neo-PAP and human PAP II. Asterisks indicate NLSs, nonconsensus cdk sites are in bold, consensus cdk sites are in bold and are underlined, plus signs indicate conserved amino acids, and minus signs indicate that no amino acid is present.
FIG. 2
Neo-PAP has nonspecific polyadenylation activity. (A) PAP I and neo-PAP proteins utilized in subsequent experiments were resolved on SDS–8% polyacrylamide gel electrophoresis and were Coomassie stained. Lanes 1 and 2, 1.2 μg of recombinant PAP I and neo-PAP expressed in and purified from E. coli. (B) Efficiencies of incorporation of α-32P-labeled nucleotides. The relative amounts of incorporated nucleotides [α-32P]ATP and [α-32P]GTP were measured. Assay conditions are described in Materials and Methods. (C) Increasing amounts (1, 2.5, 10, and 50 ng) of PAP I (lanes 2 through 5) and neo-PAP (lanes 7 through 10) were assayed in a nonspecific polyadenylation assay using a 172-nucleotide 32P-labeled RNA substrate. RNA products were resolved by denaturing polyacrylamide gel electrophoresis.
FIG. 3
Neo-PAP has specific polyadenylation and cleavage activity. (A) Recombinant PAP I, neo-PAP, or purified CPSF, alone or in the indicated combinations, was added to reaction mixtures containing either wild-type (wt; AAUAAA) or mutant (pm; AAAAAA) 32P-labeled pG3SVL-A pre-RNA. (B) Increasing amounts (5 and 20 ng) of PAP I (lanes 2 and 3) or neo-PAP (lanes 4 and 5) were assayed in a reconstitution cleavage assay (see Materials and Methods). Arrows indicate the positions of upstream (5′) and downstream (3′) cleavage products. In both panels A and B, RNA products were resolved by denaturing polyacrylamide gel electrophoresis.
FIG. 4
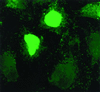
Neo-PAP localizes exclusively to the nucleus. After a 20-h transfection, HeLa cells were stained with a fluorescein isothiocyanate-conjugated anti-HA MAb and examined using immunofluorescence microscopy. Magnification, ×1,400.
FIG. 5
Neo-PAP and classic PAP appear to be differentially phosphorylated. Western blotting with an anti-HA epitope antibody was performed on extracts of 293 cells that were not transfected (“none”) or transfected with plasmids encoding green fluorescent protein (GFP), HA-PAP II, or HA-neoPAP. Some lysates (indicated with a plus sign) were treated with potato acid phosphatase prior to loading onto SDS–4 to 20% polyacrylamide gel electrophoresis. Cell equivalents were 1.6 × 105 per lane. Results are representative of three separate experiments.
FIG. 6
Neo-PAP and PAP are both overexpressed in human cancers but have distinct splicing patterns. Northern blots containing 10 μg of total RNA/lane (upper right and left panels) or approximately 2 μg of poly(A)+ RNA/lane (lower right and left panels) were hybridized with a neo-PAP probe followed by β-actin and then stripped and reprobed with PAP, followed again with β-actin. Lane 1, 1087-mel; 2, 1532-CPTX; 3, 1535-CPTX; 4, 1542-CPTX; 5, CY13; 6, LoVo; 7, SW480; 8, 293 cells; 9, 1087-EBV; 10, 1087 PBL; 11, 1532 PBL; 12, 1535 PBL; 13, brain; 14, colon; 15, heart; 16, kidney; 17, liver; 18, lung; 19, muscle; 20, placenta; 21, small intestine; 22, spleen; 23, stomach; 24, testis. Blots probed with neo-PAP or PAP were exposed to film for 67 to 72 h or with β-actin for 2 to 2.5 h. Trans., transformed cells.
FIG. 7
Expression of neo-PAP in testis and other normal tissues assessed by RT-PCR. Products of RT-PCR with primers specific for neo-PAP or β-actin were stained with ethidium bromide and were electrophoresed on a 0.8% agarose gel.
References
- Bard J, Zhelkovsky A M, Helmling S, Earnest T N, Moore C L, Bohm A. Structure of yeast poly(A) polymerase alone and in complex with 3′-dATP. Science. 2000;289:1346–1349. - PubMed
- Colgan D F, Manley J L. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 1997;11:2755–2766. - PubMed
- Colgan D F, Murthy K G K, Prives C, Manley J L. Cell-cycle related regulation of poly(A) polymerase by phosphorylation. Nature. 1996;384:282–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous