Nerve growth factor activates persistent Rap1 signaling in endosomes - PubMed (original) (raw)
Nerve growth factor activates persistent Rap1 signaling in endosomes
C Wu et al. J Neurosci. 2001.
Abstract
We investigated a role for endogenous Rap1, a small monomeric GTP-binding protein of the Ras family, in nerve growth factor (NGF) signaling in PC12 cells. Although both epidermal growth factor (EGF) and NGF caused transient activation of Ras, only NGF induced the activation of Rap1. Moreover, Rap1 activation was sustained for hours, an effect that matched the sustained activation of the mitogen-activated protein kinase (MAPK) pathway. To investigate the molecular basis for Rap1 activation, we examined complexes containing C3G, a guanine nucleotide exchange factor for Rap1, and CrkL, an adapter protein known to influence Rap1 signaling. NGF induced the formation of a long-lived complex containing C3G/CrkL/Shp2/Gab2/TrkA. Linking the complex to Rap1 activation, we coprecipitated activated TrkA and activated MAPK with activated Rap1 in NGF-treated cells. Confocal microscopy and subcellular fractionation showed that activated Rap1 and the other proteins of the signaling complex were present in endosomes. Pretreatment of PC12 cells with brefeldin A (BFA), which disrupts the Golgi and endosomal compartments, had little effect on Ras activation but strongly inhibited NGF-induced Rap1 activation and continuing MAPK activation. We propose that endosomes are a site from which NGF induces the prolonged activation of Rap1 and MAPK.
Figures
Fig. 1.
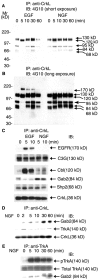
Analysis of endogenous Ras and Rap1 activation in EGF- and NGF-treated cells. Equal numbers of serum-starved PC12 cells were treated with either EGF (50 ng/ml) or NGF (50 ng/ml) for the indicated time intervals or the vehicle control (0 min).A, The cells were rinsed and lysed in RIPA buffer. Proteins (40 μg) from each cell lysate were separated on SDS-PAGE and analyzed by immunoblotting. The blot was probed with a rabbit antibody that specifically recognizes phosphorylated MAPK (top). Then the blot was reprobed with a mouse antibody against MAPK2 (bottom). B, RasGTPwas precipitated with the C-RafRBD/GST fusion protein. The fusion protein was prebound to glutathione–agarose beads before incubation with cell lysates. After incubation the beads were washed and boiled in SDS-PAGE sample buffer. The samples were separated on 12.5% SDS-PAGE, and the proteins were transferred onto PVDF membrane. The membrane was probed with a mouse antibody to Ras. C, The amounts of Rap1GTP that followed EGF and NGF treatment were assayed as in B, except that the RalGDSRBD/GST fusion proteins were used to precipitate Rap1GTP. The resulting blot was probed with a mouse antibody to Rap1.D, Cells were pretreated with either vehicle (−) or 0.5 μ
m
K252a (+) for 15 min at 37°C before treatment with NGF (50 ng/ml) for the indicated times. Activated Rap1 was assayed as in C. E, Serum-starved 6-24 cells were treated with NGF (50 ng/ml) for the indicated time intervals or the vehicle control (0 min). The amount of Rap1GTP was assayed as in C. All blots were visualized with SuperSignal; the results shown are representative of at least three independent experiments.
Fig. 2.
bFGF caused persistent activation of endogenous Rap1 and MAPK in PC12 cells. Equal numbers of serum-starved PC12 cells were treated with bFGF (50 ng/ml) for the indicated time intervals or the vehicle control (0 min), and activation of Ras (A) or Rap1 (B) was assayed as in Figure 1. C, Equal numbers of serum-starved PC12 cells were treated with bFGF (50 ng/ml) for the indicated time intervals or the vehicle control (0 min). Cells were rinsed and then lysed in RIPA buffer as in Figure 1. Proteins (40 μg) from each cell lysate were separated on SDS-PAGE and analyzed by immunoblotting. The blot was probed with a rabbit antibody against phosphorylated MAPK. Then the blot was reprobed with a rabbit antibody to total MAPK. All blots were visualized with SuperSignal; the results shown are representative of at least three independent experiments.
Fig. 3.
C3G was tyrosine phosphorylated constitutively and formed a complex with CrkL in PC12 cells. Equal numbers of serum-starved PC12 cells were treated with EGF (50 ng/ml;A) and NGF (50 ng/ml; B) for the indicated time intervals or the vehicle control (0 min). Cells were washed and then lysed as described in Materials and Methods. The supernatants were incubated with 4 μg of rabbit IgGs against C3G (A, B). C, Equal numbers of serum-starved PC12 cells were treated with EGF (50 ng/ml) or NGF (50 ng/ml) for the indicated time intervals or the vehicle control (0 min). Cells were washed and then lysed. The supernatants were incubated with 4 μg of mouse IgGs against phosphotyrosine (4G10). All immunoprecipitates were analyzed by 10% SDS-PAGE and immunoblotting. The resulting blots were probed with the specified antibody. All blots were visualized with SuperSignal; the results shown are representative of at least three independent experiments.
Fig. 4.
Different CrkL-containing complexes were induced by NGF and EGF treatment. Equal numbers of serum-starved PC12 cells were treated with either EGF (50 ng/ml) or NGF (50 ng/ml) for the indicated time intervals or the vehicle control (0 min). The cells were rinsed, lysed, and immunoprecipitated with 4 μg of rabbit antibodies to CrkL. The immunoprecipitates were separated on a 7.5% SDS-PAGE and analyzed by immunoblotting with the indicated antibodies.A, A short exposure of a blot probed with 4G10 to detect tyrosine-phosphorylated proteins. B, A longer exposure of A. EGF treatment rapidly changed the proteins associated with CrkL. NGF recruited an 84 kDa protein to CrkL.C, The CrkL immunoprecipitate was probed with antibodies to a number of signaling protein molecules. After EGF treatment a complex containing C3G/CrkL/Shp2/Cbl/EGFR was formed. In NGF-treated cells a complex was formed that contained C3G/CrkL/Shp2/Gab2/TrkA.D, CrkL was immunoprecipitated as in C, except that the cells were treated with NGF (50 ng/ml) for the indicated time intervals or the vehicle control (0 min). The immunoprecipitates were separated on SDS-PAGE, and the blot was probed with antibodies to Gab2, TrkA, and CrkL. In NGF-treated cells Gab2 and TrkA were both present in a complex with CrkL for the duration of the experiment. E, Cells were treated with NGF (50 ng/ml) for the indicated time intervals or the vehicle control (0 min), and TrkA was immunoprecipitated with MCTrk antibodies. The immunoprecipitates were separated on 10% SDS-PAGE, and the blot was probed with the following antibodies: pTrkA (pY490), total TrkA (MCTrk), and Gab2. All blots were visualized with SuperSignal; the results shown are representative of at least three independent experiments.
Fig. 5.
NGF treatment caused the association of activated TrkA with activated Rap1. Equal numbers of serum-starved PC12 cells were treated with NGF (50 ng/ml) or the vehicle control for 30 min. Rap1GTP was precipitated as described in Figure1_C_. The resulting precipitates were washed and separated by a 7.5% SDS-PAGE and transferred onto a PVDF membrane. The blot was probed with a rabbit antibody to either phosphorylated TrkA (pY490) or activated MAPK. All blots were visualized with SuperSignal; the results shown are representative of at least three independent experiments.
Fig. 6.
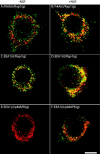
Analysis of the subcellular localization of components of the Rap1 signaling pathway by indirect immunofluorescence. PC12 cells were cultured for 24–48 hr on cover glasses that were coated with Matrigel. Cells were serum-starved and were treated with NGF (50 ng/ml; B, D, F) or the vehicle control (A, C, E) for 30 min. Then the cells were rinsed, fixed with 100% methanol, and processed for indirect immunofluorescence, as described in Materials and Methods. The primary antibodies were a rabbit antibody to Rap1 (1:250) and a mouse antibody to TrkA (1:400; A, B); a rabbit antibody to Rap1 (1:250) and a mouse antibody to EEA1 (1:120; C, D); a rabbit antibody to pMAPK (1:120) and a mouse antibody to EEA1 (1:120;E, F). Alexa 568 goat anti-mouse IgG conjugates and Alexa 488 goat anti-rabbit IgG conjugates were used to visualize the primary antibodies. g, Green fluorescence signal;r, red fluorescence signal. Colocalization of antigens is denoted by the yellow fluorescence signal. Scale bar, 10 μm.
Fig. 7.
Activation of Rap1 in endosomal fractions.A, Postnuclear supernatants generated from equal numbers of serum-starved PC12 cells were fractionated by centrifugation, using a step gradient density system as described in Materials and Methods. Four membrane fractions were collected as indicated. Total proteins from each fraction were precipitated in 7% TCA and washed in acetone. Then the pellets were dried and boiled in SDS-PAGE loading buffer. The proteins were analyzed by SDS-PAGE and immunoblotting. The blot was probed independently with antibodies to EGFR, Ras, EEA1, Rab5B, Rap1, B-Raf, Mek1, and MAPK. The molecular mass corresponding to each antigen is shown in parentheses. B, Membrane fractions were collected from serum-starved PC12 cells that were treated with either NGF (50 ng/ml) or the vehicle control for 30 min. Total proteins from each fraction were precipitated, washed, and analyzed by SDS-PAGE and immunoblotting as in A. Activated TrkA and activated MAPK were detected by using specified antibodies. To detect activated Rap1, we collected and lysed membrane fractions in fishing buffer; the amount of Rap1GTPwas assayed as described in Figure 1. All blots were visualized with SuperSignal; the results shown are representative of at least three independent experiments.
Fig. 8.
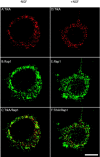
BFA pretreatment disrupted the intracellular trafficking of TrkA and Rap1. PC12 cells were cultured for 24–48 hr on cover glasses coated with Matrigel. Cells were serum-starved and were pretreated with 5 μg/ml BFA at 37°C for 15 min. Then the cells were treated with 50 ng/ml NGF (D, E, F) or the vehicle control (A, B, C) for 30 min. Cells were rinsed, fixed with 100% methanol, and processed for indirect immunofluorescence, as described in Materials and Methods. Immunostaining was performed with a mouse antibody to TrkA (1:400;A, D) and a rabbit antibody to Rap1 (1:250; B, E). Alexa 568 goat anti-mouse IgG conjugates and Alexa 488 goat anti-rabbit IgG conjugates were used to visualize TrkA and Rap1, respectively. C, A merged image of A and_B_. F, A merged image of _D_and E. Pretreatment with the BFA vehicle control (0.1% methanol) alone had no effect on the intracellular trafficking of TrkA and Rap1 after NGF treatment (data not shown). Colocalization of antigens is denoted by the yellow fluorescence signal. Scale bar, 10 μm.
Fig. 9.
Inhibition of NGF-induced Rap1 activation by BFA pretreatment. Equal numbers of serum-starved PC12 cells were pretreated with either 5 μg/ml BFA (+) or the vehicle control (−) at 37°C for 30 min. Then the cells were treated with NGF (50 ng/ml) for the indicated time intervals or the vehicle control (0 min). Activated Ras (A) and Rap1 (B) were assayed as described in Figure 1. C, PC12 cells were pretreated with either 5 μg/ml BFA (+) or the vehicle control (−) at 37°C for 15 min. Then the cells were treated with 50 ng/ml NGF for 5 or 30 min or the vehicle control (0 min). Cells were rinsed and lysed in RIPA buffer; cell lysate proteins (20 μg) were separated on 10% SDS-PAGE, transferred, and blotted with specific antibodies against activated MAPK and total MAPK. All blots were visualized with SuperSignal; the results shown are representative of at least three independent experiments.
Similar articles
- Rap1-PDZ-GEF1 interacts with a neurotrophin receptor at late endosomes, leading to sustained activation of Rap1 and ERK and neurite outgrowth.
Hisata S, Sakisaka T, Baba T, Yamada T, Aoki K, Matsuda M, Takai Y. Hisata S, et al. J Cell Biol. 2007 Aug 27;178(5):843-60. doi: 10.1083/jcb.200610073. J Cell Biol. 2007. PMID: 17724123 Free PMC article. - Trafficking the NGF signal: implications for normal and degenerating neurons.
Delcroix JD, Valletta J, Wu C, Howe CL, Lai CF, Cooper JD, Belichenko PV, Salehi A, Mobley WC. Delcroix JD, et al. Prog Brain Res. 2004;146:3-23. doi: 10.1016/s0079-6123(03)46001-9. Prog Brain Res. 2004. PMID: 14699953 - A functional dynein-microtubule network is required for NGF signaling through the Rap1/MAPK pathway.
Wu C, Ramirez A, Cui B, Ding J, Delcroix JD, Valletta JS, Liu JJ, Yang Y, Chu S, Mobley WC. Wu C, et al. Traffic. 2007 Nov;8(11):1503-20. doi: 10.1111/j.1600-0854.2007.00636.x. Epub 2007 Sep 6. Traffic. 2007. PMID: 17822405 - Biogenesis and function of the NGF/TrkA signaling endosome.
Marlin MC, Li G. Marlin MC, et al. Int Rev Cell Mol Biol. 2015;314:239-57. doi: 10.1016/bs.ircmb.2014.10.002. Epub 2014 Nov 18. Int Rev Cell Mol Biol. 2015. PMID: 25619719 Free PMC article. Review. - C3G Protein, a New Player in Glioblastoma.
Manzano S, Gutierrez-Uzquiza A, Bragado P, Cuesta AM, Guerrero C, Porras A. Manzano S, et al. Int J Mol Sci. 2021 Sep 16;22(18):10018. doi: 10.3390/ijms221810018. Int J Mol Sci. 2021. PMID: 34576182 Free PMC article. Review.
Cited by
- The many disguises of the signalling endosome.
Villarroel-Campos D, Schiavo G, Lazo OM. Villarroel-Campos D, et al. FEBS Lett. 2018 Nov;592(21):3615-3632. doi: 10.1002/1873-3468.13235. Epub 2018 Sep 17. FEBS Lett. 2018. PMID: 30176054 Free PMC article. Review. - Cell biology in neuroscience: Death of developing neurons: new insights and implications for connectivity.
Dekkers MP, Nikoletopoulou V, Barde YA. Dekkers MP, et al. J Cell Biol. 2013 Nov 11;203(3):385-93. doi: 10.1083/jcb.201306136. J Cell Biol. 2013. PMID: 24217616 Free PMC article. Review. - Coronin-1 is a neurotrophin endosomal effector that is required for developmental competition for survival.
Suo D, Park J, Harrington AW, Zweifel LS, Mihalas S, Deppmann CD. Suo D, et al. Nat Neurosci. 2014 Jan;17(1):36-45. doi: 10.1038/nn.3593. Epub 2013 Nov 24. Nat Neurosci. 2014. PMID: 24270184 Free PMC article. - Endocytosis and endosomes at the crossroads of regulating trafficking of axon outgrowth-modifying receptors.
Winckler B, Yap CC. Winckler B, et al. Traffic. 2011 Sep;12(9):1099-108. doi: 10.1111/j.1600-0854.2011.01213.x. Epub 2011 May 23. Traffic. 2011. PMID: 21535338 Free PMC article. Review. - Long-distance retrograde neurotrophic factor signalling in neurons.
Harrington AW, Ginty DD. Harrington AW, et al. Nat Rev Neurosci. 2013 Mar;14(3):177-87. doi: 10.1038/nrn3253. Nat Rev Neurosci. 2013. PMID: 23422909 Review.
References
- Anderson RGW. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. - PubMed
- Bar-Sagi D, Feramisco JR. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. - PubMed
- Beattie EC, Zhou J, Grimes ML, Bunnett NW, Howe CL, Mobley WC. A signaling endosome hypothesis to explain NGF actions: potential implications for neurodegeneration. Cold Spring Harb Symp Quant Biol. 1996;61:389–406. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources