Specific cleavage of hyper-edited dsRNAs - PubMed (original) (raw)
Specific cleavage of hyper-edited dsRNAs
A D Scadden et al. EMBO J. 2001.
Abstract
Extended double-stranded DNA (dsRNA) duplexes can be hyper-edited by adenosine deaminases that act on RNA (ADARs). Long uninterrupted dsRNA is relatively uncommon in cells, and is frequently associated with infection by DNA or RNA viruses. Moreover, extensive adenosine to inosine editing has been reported for various viruses. A number of cellular antiviral defence strategies are stimulated by dsRNA. An additional mechanism to remove dsRNA from cells may involve hyper-editing of dsRNA by ADARs, followed by targeted cleavage. We describe here a cytoplasmic endonuclease activity that specifically cleaves hyper-edited dsRNA. Cleavage occurs at specific sites consisting of alternating IU and UI base pairs. In contrast, unmodified dsRNA and even deaminated dsRNAs that contain four consecutive IU base pairs are not cleaved. Moreover, dsRNAs in which alternating IU and UI base pairs are replaced by isomorphic GU and UG base pairs are not cleaved. Thus, the cleavage of deaminated dsRNA appears to require an RNA structure that is unique to hyper-edited RNA, providing a molecular target for the disposal of hyper-edited viral RNA.
Figures
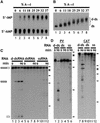
Fig. 1. (A) Quantitation of deamination. ΔKP dsRNA was deaminated to varying degrees using ADAR2. Digestion of the modified RNA using RNase P1, followed by TLC, enabled quantitation of the amount of A to I conversion. The RNAs used in the assays shown typically contained ∼40% A to I conversion (lane 8). (B) Deamination of ΔKP results in a homogenous population of RNA. As the level of deamination of ΔKP increased, there was a corresponding reduction in the mobility of the RNA on a native gel (lanes 1–8). For dsRNAs with ∼40% A to I conversion (lane 8), the RNA migrated as a relatively compact band (‘d-ds’), indicating that all RNAs in the population were modified. (C) Deaminated dsRNA is specifically cleaved. Incubation of ΔKP d-dsRNA in Xenopus oocyte extract gave rise to two discrete cleavage products (lanes 1–4). In contrast, ΔKP dsRNA was stable (lanes 5–8), and ΔKP ssRNA was degraded slowly yielding numerous products (lanes 9–12). The time course used in each assay was 0, 15, 60 and 90 min. Positions of DNA molecular weight markers are shown to the right of the figure (φX174 _Hae_III, 310, 281, 271, 234, 194, 118, and 72 nt). (D) Incubation of both PV and CAT d-dsRNAs for 90 min in Xenopus oocyte extracts also gave rise to discrete cleavage products (lanes 1–6 and 7–12, respectively). The equivalent dsRNAs were stable (compare lanes 2 and 4, and lanes 8 and 10), while degradation of the ssRNAs gave more numerous products (lanes 6 and 12).
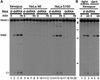
Fig. 2. (A) d-dsRNA is cleaved by a cytoplasmic ribonuclease. ΔKP d-dsRNA was specifically cleaved to give two major products when incubated in either Xenopus oocyte extract or HeLa cell S100 (lanes 1–4 and 13–16, respectively). In contrast, ΔKP d-dsRNA was predominantly stable in HeLa cell nuclear extract (NE; lanes 5–8). The minor amount of cleavage detected could probably be accounted for by cytoplasmic contamination of the nuclear extract. ΔKP dsRNA was stable in both HeLa cell S100 and NE extracts (lanes 17–20 and 9–12, respectively). A cytoplasmic activity thus appears to be responsible for cleavage of d-dsRNA. The time course used in each assay was 0, 15, 60 and 90 min. (B) ΔKP d-dsRNA does not undergo photocleavage. Cleavage of ΔKP d-dsRNA in Xenopus oocyte extract was identical in the light and dark (compare lanes 1–3 and 4–6, respectively). This indicated that photocleavage was not responsible for the observed cleavage. The time course used in each assay was 0, 30 and 60 min.
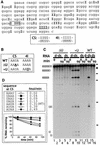
Fig. 3. (A) Identification of the sites of deamination in ΔKP d-dsRNA. Cloned RT–PCR products derived from a ΔKP d-dsRNA template deaminated to 40% were sequenced to identify positions of deamination. The sequence shown corresponds to the sense strand of ΔKP, and positions of A to I conversion are indicated by ‘I’. This sequence is typical of a number of sequenced clones. Cleavage of the d-dsRNA occurred at the sequence IIUI (boxed; referred to as CS). The sequence underlined (IIII) is referred to as 4I. The double-stranded sequence of the CS and 4I sites is indicated. (B) Two mutants of ΔKP were generated, where mutations were made in either the CS (ΔU mutant) or 4I (+U mutant) sequences, as shown (sense strand). ΔU potentially contained no cleavage sites while +U potentially contained two cleavage sites (compared with the single site in wild-type (WT) ΔKP). (C) Alternating IU and UI base pairs results in cleavage. ΔKP d-dsRNA (WT) was cleaved at the sequence CS in Xenopus oocyte extract to give two products (lane 14). In contrast, incubation of ΔU d-dsRNA yielded no cleavage products (lanes 1–4). Cleavage of +U d-dsRNA resulted in several products that corresponded to cleavage at both the CS and modified 4I sequences (lanes 7–10). The equivalent WT, ΔU and +U dsRNAs were stable (lanes 16, 6 and 12). The time course used to assay ΔU and +U d-dsRNAs was 0, 15, 60 and 90 min. (D) Mutants of ΔKP were generated where the WT CS sequence (AAUA) was replaced by the given sequences. The corresponding d-dsRNAs were incubated in Xenopus oocyte extract, and the cleavage was analysed by phosphoimaging. The initial rate of cleavage for each mutant (fmol full-length RNA cleaved/min) was calculated, as given in the table. The graph shows the cleavage of each d-dsRNA over a 90 min time course. These data indicate that sequences which potentially contain different arrangements of IU and UI base pairs are not cleaved with equal efficiently following hyper-editing.
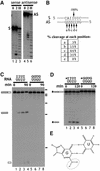
Fig. 4. (A) Cleavage of short synthetic dsRNA substrates. Short synthetic dsRNAs that contained the CS sequence were used to analyse cleavage. Incubation of short dsRNAs, 5′ end-labelled on either the sense (S) or antisense strand (AS), in Xenopus oocyte extract indicated that both RNA strands were cleaved (lanes 3 and 6). Electrophoresis alongside an alkaline hydrolysis ladder (H; lanes 1 and 4) enabled mapping of the cleavage sites on the sense and antisense strands. (B) Mapping the cleavage sites. The short synthetic RNAs were cleaved at the positions indicated. The sense strand was cleaved at one major position, 5′ of a U residue, and within the sequence of alternating IU and UI base pairs. In contrast, the antisense strand was cleaved at five positions, where ∼90% of the cleavage occurred 5′ of U residues. (C) Alternating GU and UG base pairs are not cleaved. ΔKP dsRNA was synthesized where the sequence of the cleavage site comprised GU and UG base pairs (as indicated above gel). In contrast with ΔKP d-dsRNA, this dsRNA was not cleaved when incubated in Xenopus oocyte extract (compare lanes 1–4 and 5–8). The time course used in each assay was 0, 15, 60 and 90 min. (D) Short dsRNAs that contained either alternating IU and UI base pairs or GU and UG base pairs (indicated above gel) were incubated in Xenopus oocyte extract. The time course used in each assay was 0, 30, 60 and 120 min. Each of the RNAs shown is labelled on the sense strand (indicated by *). While the dsRNAs containing alternating IU and UI base pairs were cleaved (lanes 1–4), the dsRNAs that contained GU and UG base pairs were stable (lanes 5–8). Thus, substitution of guanosine residues for inosine residues in the cleavage site inhibits cleavage of the RNA. (E) A non-Watson–Crick GU wobble base pair. An IU wobble base pair is isosteric with the GU base pair, but lacks the exocyclic amine group (circled) which projects into the minor groove.
Similar articles
- Cleavage of dsRNAs hyper-edited by ADARs occurs at preferred editing sites.
Scadden AD, O'Connell MA. Scadden AD, et al. Nucleic Acids Res. 2005 Oct 27;33(18):5954-64. doi: 10.1093/nar/gki909. Print 2005. Nucleic Acids Res. 2005. PMID: 16254076 Free PMC article. - The RISC subunit Tudor-SN binds to hyper-edited double-stranded RNA and promotes its cleavage.
Scadden AD. Scadden AD. Nat Struct Mol Biol. 2005 Jun;12(6):489-96. doi: 10.1038/nsmb936. Epub 2005 May 15. Nat Struct Mol Biol. 2005. PMID: 15895094 - Unique conformational dynamics and protein recognition of A-to-I hyper-edited dsRNA.
Müller-Hermes C, Piomponi V, Hilber S, Asami S, Kreutz C, Bussi G, Sattler M. Müller-Hermes C, et al. Nucleic Acids Res. 2025 Jun 20;53(12):gkaf550. doi: 10.1093/nar/gkaf550. Nucleic Acids Res. 2025. PMID: 40568935 Free PMC article. - Adenosine deaminases acting on RNA (ADARs) are both antiviral and proviral.
Samuel CE. Samuel CE. Virology. 2011 Mar 15;411(2):180-93. doi: 10.1016/j.virol.2010.12.004. Epub 2011 Jan 5. Virology. 2011. PMID: 21211811 Free PMC article. Review. - Mapping the dsRNA World.
Reich DP, Bass BL. Reich DP, et al. Cold Spring Harb Perspect Biol. 2019 Mar 1;11(3):a035352. doi: 10.1101/cshperspect.a035352. Cold Spring Harb Perspect Biol. 2019. PMID: 30824577 Free PMC article. Review.
Cited by
- Regulation of Human Endonuclease V Activity and Relocalization to Cytoplasmic Stress Granules.
Nawaz MS, Vik ES, Berges N, Fladeby C, Bjørås M, Dalhus B, Alseth I. Nawaz MS, et al. J Biol Chem. 2016 Oct 7;291(41):21786-21801. doi: 10.1074/jbc.M116.730911. Epub 2016 Aug 29. J Biol Chem. 2016. PMID: 27573237 Free PMC article. - VIRGO: visualization of A-to-I RNA editing sites in genomic sequences.
Distefano R, Nigita G, Macca V, Laganà A, Giugno R, Pulvirenti A, Ferro A. Distefano R, et al. BMC Bioinformatics. 2013;14 Suppl 7(Suppl 7):S5. doi: 10.1186/1471-2105-14-S7-S5. Epub 2013 Apr 22. BMC Bioinformatics. 2013. PMID: 23815474 Free PMC article. - Pronounced instability of tandem IU base pairs in RNA.
Serra MJ, Smolter PE, Westhof E. Serra MJ, et al. Nucleic Acids Res. 2004 Mar 22;32(5):1824-8. doi: 10.1093/nar/gkh501. Print 2004. Nucleic Acids Res. 2004. PMID: 15037659 Free PMC article. - Tudor staphylococcal nuclease is a structure-specific ribonuclease that degrades RNA at unstructured regions during microRNA decay.
Li CL, Yang WZ, Shi Z, Yuan HS. Li CL, et al. RNA. 2018 May;24(5):739-748. doi: 10.1261/rna.064501.117. Epub 2018 Feb 13. RNA. 2018. PMID: 29440319 Free PMC article. - A genome-wide map of hyper-edited RNA reveals numerous new sites.
Porath HT, Carmi S, Levanon EY. Porath HT, et al. Nat Commun. 2014 Aug 27;5:4726. doi: 10.1038/ncomms5726. Nat Commun. 2014. PMID: 25158696 Free PMC article.
References
- Ausubel F.M., Brent,R., Kingston,R.E., Moore,D.D., Seidman,J.G., Smith,J.A. and Struhl,K. (1994) Current Protocols in Molecular Biology. John Wiley & Sons, New York, NY.
- Bass B.L. (2000) Double-stranded RNA as a template for gene silencing. Cell, 101, 235–238. - PubMed
- Bass B.L. and Weintraub,H. (1988) An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell, 55, 1089–1098. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources