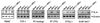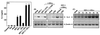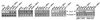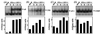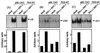Requirements for the induction of interleukin-6 by herpes simplex virus-infected leukocytes - PubMed (original) (raw)
Requirements for the induction of interleukin-6 by herpes simplex virus-infected leukocytes
S R Paludan. J Virol. 2001 Sep.
Abstract
Cytokines play important roles in the clearance of herpes simplex virus (HSV) infections and in virus-induced immunopathology. One cytokine known to contribute to resistance against HSV is interleukin-6 (IL-6). Here we have investigated virus-cell interactions responsible for IL-6 induction by HSV in leukocytes. Both HSV type 1 and type 2 are potent inducers of IL-6, and this phenomenon is augmented in the presence of gamma interferon. The ability to induce IL-6 is dependent on de novo protein synthesis and is sensitive to UV irradiation of the virus. Virus mutants lacking the virion-transactivating protein VP16 or any of the immediate-early proteins ICP0, ICP4, or ICP27 displayed unaltered capacities to induce IL-6. However, wild-type virus was unable to induce IL-6 in a macrophage cell line overexpressing a mutant of double-stranded RNA-activated protein kinase (PKR). This suggests a role for PKR in HSV-induced IL-6 expression. HSV infection led to enhanced binding to the kappaB, CRE, and AP-1 sites of the IL-6 promoter, and inhibitors against NF-kappaB and the p38 kinase strongly reduced accumulation of IL-6 mRNA in infected cells. Moreover, macrophage cell lines expressing dominant negative mutants of IkappaBalpha and p38 responded to HSV-1 infection with reduced IL-6 expression compared to the control-vector-transfected cell line. The results show that induction of IL-6 by HSV in leukocytes is dependent on PKR and cellular signaling through NF-kappaB and a p38-dependent pathway.
Figures
FIG. 1
Expression of IL-6 mRNA in various human and murine leukocyte populations. Primary cells were isolated as described in Materials and Methods. The primary cells and cell lines were seeded and left overnight to settle. The cells were infected with 3 × 106 PFU of HSV-1 (KOS) per ml and stimulated with 10 IU of human or murine IFN-γ per ml. After 4 h of treatment, total RNA was isolated and subjected to RT-PCR using oligo(dT)15 RT priming and PCR primers specific for murine β-actin, human IL-6, and murine IL-6. PBMC, peripheral blood mononuclear cells; PC, peritoneal cells; TG, thioglycolate.
FIG. 2
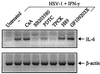
Characterization of IL-6 induction by HSV-kinetics and effects of cycloheximide, anti-TNF-α, and UV-irradiation of the virus. RAW 264.7 cells were seeded and left overnight to settle. (A) The cells were treated with 3 × 106 PFU of HSV-1 (KOS) per ml, 3 × 106 PFU of HSV-2 (MS) per ml, and 10 IU of IFN-γ per ml. After 24 h, supernatants were harvested and analyzed for IL-6 by ELISA. The results are shown as means ± standard errors of the means. (B) The cells were treated as indicated with the following concentrations: 3 × 106 PFU of HSV-1 (KOS) per ml or equivalent amount of UV-irradiated virus, 10 IU of IFN-γ per ml, 1,000 U of neutralizing anti-TNF-α per ml, 10 μg of CHX per ml. After 4 h of treatment total RNA was isolated. (C) The cells were treated with 3 × 106 PFU of HSV-1 (KOS) per ml and 10 IU of IFN-γ per ml. After the indicated time points total RNA was isolated. (B and C) The RNA was subjected to RT-PCR using oligo(dT)15 RT-priming and PCR primers specific for murine β-actin and IL-6.
FIG. 3
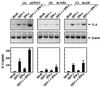
Induction of IL-6 by mutant viruses in wild-type cells and by wild-type HSV-1 in macrophages unable to activate PKR in response to double-stranded RNA. (A) RAW 264.7 cells were seeded and left overnight to settle. The cells were treated with 10 IU of IFN-γ per ml and 3 × 106 PFU of wild-type or mutant HSV-1 per ml. After 4 h of treatment, total RNA was isolated and subjected to RT-PCR using oligo(dT)15 RT-priming and PCR primers specific for murine β-actin and IL-6. (B) The RAW 264.7-derived cell lines pBK-CMV and PKR-M7 were seeded as above. Sixteen hours later the cells were treated with 10 IU of IFN-γ per ml and 3 × 106 PFU of HSV-1 (KOS) per ml. After 4 h of treatment, total RNA was extracted and analyzed for IL-6 and β-actin by RT-PCR.
FIG. 4
Binding activity to κB, CRE, AP-1, and C/EBP sites of the murine IL-6 promoter. RAW 264.7 cells were treated with 10 IU of IFN-γ per ml and infected with 3 × 106 PFU of HSV-1 (KOS) per ml for the indicated time periods, and nuclear extracts were prepared. The extracts were tested for binding to the following probes: κB (A), CRE (B), AP-1 (C), and C/EBP (D). Five micrograms of nuclear proteins and 20,000 cpm of 32P-labeled probe were used per reaction. The binding was quantified by densitometric measurements of the autoradiographs. The results are shown as histograms using arbitrary units.
FIG. 5
PKR dependency of binding activity on the κB, CRE, and AP-1 sites of the murine IL-6 promoter. RAW-pBK-CMV or RAW-PKR-M7 cells were mock infected or treated with 10 IU of IFN-γ per ml and 3 × 106 PFU of HSV-1 (KOS) per ml. Nuclear extracts were prepared 3.5 h later, and the extracts were tested for binding to κB (A), CRE (B), and AP-1 (C) probes. Five micrograms of nuclear proteins and 20,000 cpm of 32P-labeled probe were used per reaction. The binding was quantified by densitometric measurements of the autoradiographs. The results are shown as histograms using arbitrary units.
FIG. 6
Effects of chemical inhibitors on HSV-induced IL-6 expression. RAW 264.7 cells were seeded and left overnight to settle. The cells were treated with inhibitors and left for 15 min prior to stimulation with 10 IU of IFN-γ per ml and infection with 3 × 106 PFU of HSV-1 (KOS) per ml. Four hours later, total RNA was extracted and IL-6 and β-actin were detected by RT-PCR using primers specific for the two mRNA species.
FIG. 7
Induction of IL-6 by HSV-1 in macrophages stably transfected with dominant negative IκBα and p38. RAW 264.7-derived cell lines with the following characteristics were generated: pcDNA3 (A), dominant negative IκBα (B), dominant negative p38V (C). The cells were seeded, infected with 3 × 106 PFU of HSV-1 (KOS) per ml, and stimulated with 10 IU of IFN-γ per ml. After 4 h, cells to be analyzed for mRNA expression were lysed and total RNA was extracted. IL-6 and β-actin were detected by RT-PCR using primers specific for the two mRNA species (upper panel). Other cells were left for 24 h, at which point supernatants were harvested and analyzed for IL-6 protein by ELISA. The results (lower panel) are shown as means ± standard errors of the means.
Similar articles
- Herpes simplex virus selectively induces expression of the CC chemokine RANTES/CCL5 in macrophages through a mechanism dependent on PKR and ICP0.
Melchjorsen J, Pedersen FS, Mogensen SC, Paludan SR. Melchjorsen J, et al. J Virol. 2002 Mar;76(6):2780-8. doi: 10.1128/jvi.76.6.2780-2788.2002. J Virol. 2002. PMID: 11861845 Free PMC article. - Viral activation of macrophages through TLR-dependent and -independent pathways.
Malmgaard L, Melchjorsen J, Bowie AG, Mogensen SC, Paludan SR. Malmgaard L, et al. J Immunol. 2004 Dec 1;173(11):6890-8. doi: 10.4049/jimmunol.173.11.6890. J Immunol. 2004. PMID: 15557184 - Herpes simplex virus type 2 induces secretion of IL-12 by macrophages through a mechanism involving NF-kappaB.
Malmgaard L, Paludan SR, Mogensen SC, Ellermann-Eriksen S. Malmgaard L, et al. J Gen Virol. 2000 Dec;81(Pt 12):3011-3020. doi: 10.1099/0022-1317-81-12-3011. J Gen Virol. 2000. PMID: 11086132 - The relationship between interleukin-6 and herpes simplex virus type 1: implications for behavior and immunopathology.
Baker M, Noisakran S, Gebhardt BM, Kriesel JD, Carr DJ. Baker M, et al. Brain Behav Immun. 1999 Sep;13(3):201-11. doi: 10.1006/brbi.1999.0572. Brain Behav Immun. 1999. PMID: 10469522 Review.
Cited by
- Protein kinase R-dependent regulation of interleukin-10 in response to double-stranded RNA.
Chakrabarti A, Sadler AJ, Kar N, Young HA, Silverman RH, Williams BRG. Chakrabarti A, et al. J Biol Chem. 2008 Sep 12;283(37):25132-25139. doi: 10.1074/jbc.M804770200. Epub 2008 Jul 14. J Biol Chem. 2008. PMID: 18625702 Free PMC article. - Regulation of T-type Ca2+ channel expression by interleukin-6 in sensory-like ND7/23 cells post-herpes simplex virus (HSV-1) infection.
Zhang Q, Hsia SC, Martin-Caraballo M. Zhang Q, et al. J Neurochem. 2019 Oct;151(2):238-254. doi: 10.1111/jnc.14697. Epub 2019 May 15. J Neurochem. 2019. PMID: 30888683 Free PMC article. - Transient blocking of NK cell function with small molecule inhibitors for helper dependant adenoviral vector-mediated gene delivery.
Ankathatti Munegowda M, Hu J. Ankathatti Munegowda M, et al. Cell Biosci. 2015 Jun 11;5:29. doi: 10.1186/s13578-015-0023-0. eCollection 2015. Cell Biosci. 2015. PMID: 26085921 Free PMC article. - Effect of a single point mutation on equine herpes virus 9 (EHV-9) neuropathogenicity after intranasal inoculation in a hamster model.
Saleh AG, Anwar SI, Abas OM, Abd-Ellatieff HA, Nasr M, Saleh I, Fukushi H, Yanai T. Saleh AG, et al. J Vet Med Sci. 2017 Aug 18;79(8):1426-1436. doi: 10.1292/jvms.17-0076. Epub 2017 Jul 15. J Vet Med Sci. 2017. PMID: 28717112 Free PMC article. - Modulation of the Tissue Expression Pattern of Zebrafish CRP-Like Molecules Suggests a Relevant Antiviral Role in Fish Skin.
Bello-Perez M, Adamek M, Coll J, Figueras A, Novoa B, Falco A. Bello-Perez M, et al. Biology (Basel). 2021 Jan 22;10(2):78. doi: 10.3390/biology10020078. Biology (Basel). 2021. PMID: 33498981 Free PMC article.
References
- Akira S, Kishimoto T. NF-IL6 and NF-kB in cytokine gene regulation. Adv Immunol. 1997;65:1–46. - PubMed
- Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. - PubMed
- Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources