In vivo generation and characterization of a soluble form of the Semliki forest virus fusion protein - PubMed (original) (raw)
In vivo generation and characterization of a soluble form of the Semliki forest virus fusion protein
Y E Lu et al. J Virol. 2001 Sep.
Abstract
During infection of host cells, a number of enveloped animal viruses are known to produce soluble forms of viral membrane glycoproteins lacking the transmembrane domain. The roles of such soluble glycoproteins in viral life cycles are incompletely understood, but in several cases they are believed to modulate host immune response and viral pathogenesis. Semliki Forest virus (SFV) is an enveloped alphavirus that infects cells through low-pH-dependent fusion and buds from the plasma membrane. Fusion is mediated by the E1 subunit of the SFV spike protein. Previous studies described the in vivo generation of E1s, a truncated soluble form of E1, under conditions in which budding is inhibited in mammalian host cells. We have here examined the properties of E1s generation and the biological activity of E1s. E1s cleavage required spike protein transport out of the endoplasmic reticulum and was independent of virus infection. Cell surface E1 efficiently acted as a precursor for E1s. E1s generation was strongly pH dependent in BHK cells, with optimal cleavage at a pH of < or =7.0, conditions that inhibited the budding of SFV but not the budding of the rhabdovirus vesicular stomatitis virus. The pH dependence of E1s production and SFV budding was unaffected by the stability of the spike protein dimer but was a function of the host cell. Similar to the intact virus and in vitro-generated E1 ectodomain, treatment of E1s at low pH in the presence of target membranes triggered specific acid-dependent conformational changes. Thus, under a variety of conditions, SFV-infected cells can produce a soluble form of E1 that is biologically active.
Figures
FIG. 1
Evaluation of E1 cleavage in the absence of virus infection. COS-7 cells were transfected with pCB3-wt or pCB3-G91D DNA encoding SFV structural proteins, as described in Materials and Methods. Forty-eight hours posttransfection, cells were pulse-labeled with 200 μCi of [35S]Met-Cys/ml for 15 min and chased for 0 or 2 h at 37°C. The medium samples (M) were collected, and the cells (C) were lysed. The radiolabeled spike proteins released in the medium and remaining in the cells were quantitated by immunoprecipitation with a polyclonal antibody against the spike proteins, followed by SDS–12% PAGE and fluorography. The data shown are a representative example of two experiments.
FIG. 2
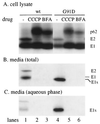
Effect of transport inhibitors on E1 cleavage. wt or G91D infectious RNAs were transcribed in vitro and transfected into BHK cells. The cells were incubated at 37°C for 6 h, pulse-labeled with 100 μCi of [35S]Met-Cys/ml for 5 min at 37°C, and then chased for 2 h at 37°C. To follow the effect of transport inhibitors on E1 cleavage, parallel cultures of cells were incubated with 50 μg of CCCP/ml or 5 μg of BFA/ml on ice for 10 min after the labeling and then chased at 37°C in the presence of these transport inhibitors. The media were collected, and the cells were lysed. TX-114 was added to the medium, and one aliquot of the medium sample was phase separated to yield aqueous and detergent phases which contained soluble and membrane proteins, respectively. Viral spike proteins in the medium (B), the aqueous phase of the medium (C), and the remaining cell lysates (A) were quantitated by immunoprecipitation with a polyclonal antibody against the spike proteins followed by SDS-PAGE analysis. The data shown are a representative example of two experiments. Note that the small amount of wt viral proteins present in the aqueous phase (panel C, lane 1) was not observed in a separate experiment.
FIG. 3
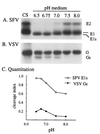
pH dependence of SFV and VSV glycoprotein cleavage. BHK cells were infected with either SFV or VSV for a total of 5 h, radiolabeled for 15 min with [35S]Met-Cys at 100 μCi/ml for SFV and 200 μCi/ml for VSV, and chased for 45 min, and the cell surface proteins were derivatized with biotin (41). The biotinylated cells were then incubated for 3 h either on ice at pH 8.0 (referred to as time zero) or at 37°C at the indicated pH. The media were collected, and TX-100 was added to a final concentration of 1%. Biotinylated proteins in one-quarter of the medium were quantitatively retrieved with mag-SA as described in Materials and Methods. The cells that were incubated on ice were lysed in a TX-100-containing buffer, and one-quarter of the lysate was retrieved to quantitate the level of biotinylated cell surface proteins at time zero (labeled CS). Retrieved samples were analyzed by SDS-PAGE (A and B) and quantitated by phosphorimaging (C). To compare the pH dependence of glycoprotein cleavage, release of SFV E1s and VSV Gs was expressed as a cleavage index, calculated as described in Materials and Methods.
FIG. 4
pH dependence of SFV and VSV budding. BHK cells were infected with either SFV or VSV, labeled, chased, derivatized with biotin, and incubated to allow the incorporation of biotinylated viral spike protein into budded virus particles, all as described in the legend to Fig. 3. The post-biotin incubation media were collected, and the biotinylated virus particles and cleaved glycoproteins were quantitatively retrieved in the absence of detergent. The mag-SA-bound material was analyzed by SDS-PAGE (A and B). To compare the SFV and VSV budding efficiencies, the amount of E2 (SFV) or M protein (VSV) was quantitated by phosphorimaging, and budding at each pH was expressed as a percentage of the E2 or M release at pH 8.0 (C). The data are representative of four (SFV) and one (VSV) experiments.
FIG. 5
pH dependence of E1 cleavage and budding for SFV mL. BHK cells were infected with the p62 cleavage-defective mL and radiolabeled, chased, and biotin-derivatized as for Fig. 3, followed by incubation in media of the indicated pH for 60 min at 37°C. The media were collected and retrieved with mag-SA in the absence of detergent, followed by SDS-PAGE analysis (A) and quantitation by phosphorimaging (B). The pH dependence of wt SFV budding was measured in parallel and similarly quantitated (B). Virus budding efficiency at each pH was calculated as a percentage of the E2 (wt) or p62 (mL) release at pH 8.0 (B). The data are representative of four (wt SFV) or two (mL) experiments.
FIG. 6
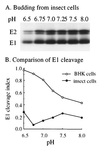
pH dependence of SFV E1 cleavage and budding in insect cells. C6/36 cells were infected with SFV at a concentration of 100 PFU/cell for a total of 6 h, pulse-labeled for 15 min with 50 μCi of [35S]Met-Cys/ml, chased for 30 min, and derivatized with biotin. The cells were then incubated at 28°C for 1 h in medium of the indicated pH to allow virus budding. Virus particles and biotinylated proteins in the medium were retrieved with mag-SA in the absence of detergent and analyzed by SDS-PAGE (A). SFV budding and cleavage in BHK cells was assayed in parallel as for Fig. 4. Although no detectable E1s was produced by the insect cells, cleavage indices for both cell types were calculated as described in Materials and Methods and compared in panel B.
FIG. 7
Production of E1s in G91D-infected BHK and insect cells. BHK or C6/36 cells were transfected with G91D infectious RNA using Lipofectamine or Cellfectin reagents, respectively. After 16 h of incubation at 28°C, cells were pulse-labeled with 200 μCi of [35S]Met-Cys/ml for 30 min at 37°C (BHK cells) or 28°C (C6/36 cells) and chased for 4 h at 28°C. The media were collected, and the cells were lysed. TX-114 was added to the medium samples, and an aliquot of each was phase separated as for Fig. 2 to yield an aqueous phase containing soluble proteins and a detergent phase containing the transmembrane forms of E1 and E2. The cell lysates, medium samples, and both phases of the media were immunoprecipitated as described in the legend to Fig. 1 and analyzed by electrophoresis on 12% acrylamide gels. To better visualize the cleaved products, the medium samples in the BHK cell panel (lanes 2, 3, and 4) were exposed twice as long as lane 1, and the medium samples in the C6/36 cell panel (lanes 3 and 4) were exposed twice as long as lanes 1 and 2. The data are representative of three experiments.
FIG. 8
Biological activity of E1s. Radiolabeled E1s or E1* was mixed with either complete liposomes (+sterol) or liposomes lacking sterol (−sterol), treated at pH 7.0 or 5.5 for 10 min, and adjusted to neutral pH. Aliquots of the samples were then treated as follows. (A) The total spike proteins in the reaction were quantitated by immunoprecipitation with a polyclonal rabbit antibody against the spike protein. (B) The samples were immunoprecipitated with MAb E1a-1, a monoclonal antibody specific for the acid conformation of E1. (C) Samples were digested with 200 μg of trypsin/ml for 15 min at 37°C to evaluate the trypsin-resistant homotrimer. All samples were then analyzed by SDS-PAGE and fluorography, and equivalent exposures of all E1s lanes and of all E1* lanes are shown. The data shown are a representative example of three separate experiments.
Similar articles
- Membrane and protein interactions of a soluble form of the Semliki Forest virus fusion protein.
Klimjack MR, Jeffrey S, Kielian M. Klimjack MR, et al. J Virol. 1994 Nov;68(11):6940-6. doi: 10.1128/JVI.68.11.6940-6946.1994. J Virol. 1994. PMID: 7933075 Free PMC article. - Formation and characterization of the trimeric form of the fusion protein of Semliki Forest Virus.
Gibbons DL, Ahn A, Chatterjee PK, Kielian M. Gibbons DL, et al. J Virol. 2000 Sep;74(17):7772-80. doi: 10.1128/jvi.74.17.7772-7780.2000. J Virol. 2000. PMID: 10933683 Free PMC article. - The domain I-domain III linker plays an important role in the fusogenic conformational change of the alphavirus membrane fusion protein.
Zheng Y, Sánchez-San Martín C, Qin ZL, Kielian M. Zheng Y, et al. J Virol. 2011 Jul;85(13):6334-42. doi: 10.1128/JVI.00596-11. Epub 2011 May 4. J Virol. 2011. PMID: 21543498 Free PMC article. - Assembly and entry mechanisms of Semliki Forest virus.
Garoff H, Wilschut J, Liljeström P, Wahlberg JM, Bron R, Suomalainen M, Smyth J, Salminen A, Barth BU, Zhao H, et al. Garoff H, et al. Arch Virol Suppl. 1994;9:329-38. doi: 10.1007/978-3-7091-9326-6_33. Arch Virol Suppl. 1994. PMID: 8032265 Review. - Mechanisms of enveloped virus entry into cells.
Kielian M, Jungerwirth S. Kielian M, et al. Mol Biol Med. 1990 Feb;7(1):17-31. Mol Biol Med. 1990. PMID: 2182968 Review.
Cited by
- A key interaction between the alphavirus envelope proteins responsible for initial dimer dissociation during fusion.
Fields W, Kielian M. Fields W, et al. J Virol. 2013 Apr;87(7):3774-81. doi: 10.1128/JVI.03310-12. Epub 2013 Jan 16. J Virol. 2013. PMID: 23325694 Free PMC article. - An alphavirus temperature-sensitive capsid mutant reveals stages of nucleocapsid assembly.
Zheng Y, Kielian M. Zheng Y, et al. Virology. 2015 Oct;484:412-420. doi: 10.1016/j.virol.2015.05.011. Epub 2015 Jun 6. Virology. 2015. PMID: 26051211 Free PMC article. - The Alphavirus Exit Pathway: What We Know and What We Wish We Knew.
Brown RS, Wan JJ, Kielian M. Brown RS, et al. Viruses. 2018 Feb 22;10(2):89. doi: 10.3390/v10020089. Viruses. 2018. PMID: 29470397 Free PMC article. Review. - Effects of membrane potential and sphingolipid structures on fusion of Semliki Forest virus.
Samsonov AV, Chatterjee PK, Razinkov VI, Eng CH, Kielian M, Cohen FS. Samsonov AV, et al. J Virol. 2002 Dec;76(24):12691-702. doi: 10.1128/jvi.76.24.12691-12702.2002. J Virol. 2002. PMID: 12438595 Free PMC article. - CHIKV strains Brazil (wt) and Ross (lab-adapted) differ with regard to cell host range and antiviral sensitivity and show CPE in human glioblastoma cell lines U138 and U251.
Hucke FIL, Bestehorn-Willmann M, Bassetto M, Brancale A, Zanetta P, Bugert JJ. Hucke FIL, et al. Virus Genes. 2022 Jun;58(3):188-202. doi: 10.1007/s11262-022-01892-x. Epub 2022 Mar 26. Virus Genes. 2022. PMID: 35347588 Free PMC article.
References
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. - PubMed
- Brewer C B. Cytomegalovirus plasmid vectors for permanent lines of polarized epithelial cells. Methods Cell Biol. 1994;43:233–245. - PubMed
- Chardin P, McCormick F. Brefeldin A: the advantage of being uncompetitive. Cell. 1999;97:153–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources