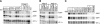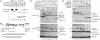Nrarp is a novel intracellular component of the Notch signaling pathway - PubMed (original) (raw)
Nrarp is a novel intracellular component of the Notch signaling pathway
E Lamar et al. Genes Dev. 2001.
Abstract
The Lin12/Notch receptors regulate cell fate during embryogenesis by activating the expression of downstream target genes. These receptors signal via their intracellular domain (ICD), which is released from the plasma membrane by proteolytic processing and associates in the nucleus with the CSL family of DNA-binding proteins to form a transcriptional activator. How the CSL/ICD complex activates transcription and how this complex is regulated during development remains poorly understood. Here we describe Nrarp as a new intracellular component of the Notch signaling pathway in Xenopus embryos. Nrarp is a member of the Delta-Notch synexpression group and encodes a small protein containing two ankyrin repeats. Nrarp expression is activated in Xenopus embryos by the CSL-dependent Notch pathway. Conversely, overexpression of Nrarp in embryos blocks Notch signaling and inhibits the activation of Notch target genes by ICD. We show that Nrarp forms a ternary complex with the ICD of XNotch1 and the CSL protein XSu(H) and that in embryos Nrarp promotes the loss of ICD. By down-regulating ICD levels, Nrarp could function as a negative feedback regulator of Notch signaling that attenuates ICD-mediated transcription.
Figures
Figure 1
Predicted protein product of Nrarp. (A) Nrarp encodes a small novel protein of 114 amino acids. Analysis using the SMART program predicts two tandem ankyrin repeats (Ankyrin Repeats 1 and 2) that constitute the carboxyl half of the protein. Nrarptr is truncated after amino acid 51. (B) BLAST homology search of the EST databases identifies rat and zebrafish homologs that are nearly identical in amino acid sequence.
Figure 2
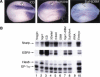
Expression of Nrarp mirrors that of other Notch pathway genes. Xenopus embryos were stained for the expression of Nrarp RNA using whole-mount in situ hybridization. (A) Nrarp expression at early neurulae stages. Shown is a dorsal view with anterior oriented to the left. Note that Nrarp RNA is expressed at high levels in the neural plate, localizing to the three bilateral domains where the Notch pathway is known to operate during the formation of the primary neurons (M, medial; I, intermediate; L, lateral). In addition, Nrarp RNA can be detected in the paraxial mesoderm (PM). (B,C) For comparison, note that the expression pattern of Nrarp is similar to that of XDelta1 (B) and ESR1 (C). (D) Expression of Nrarp RNA in a tadpole embryo stained in whole mount. Shown is a side view of the anterior end with anterior oriented to the left. Note expression in the eye (EY), neural tube (NT), and branchial arches (BA). (E) Tissue section of an embryo as in panel D shows that the expression of Nrarp RNA in the neural tube is confined to the ventricular zone where neurogenesis occurs. (F) Expression of Nrarp occurs at high levels in the outgrowing tailbud where Notch signaling is known to play a role in segmentation. High levels of Nrarp expression are apparent in the most posterior portion of the presomitic mesoderm called the tailbud domain (TBD) and in segments in a region containing prospective somites. This expression pattern in the presomitic mesoderm closely parallels that of XDelta2 and two Notch target genes, ESR4 and ESR5.
Figure 3
Nrarp expression is regulated by the CSL-dependent Notch pathway. (A) Xenopus embryos at the two-cell stage were injected with RNA encoding ICD, XSu(H)Ank, or XSu(H)DBM along with LacZ RNA as a tracer. At neural plate stages, embryos were fixed, stained for β-galactosidase activity, and analyzed for expression of Nrarp using whole-mount in situ hybridization. Shown is a dorsal view of the neural plate with anterior oriented to the left and the injected side of the embryos oriented up. Note that both ICD and XSu(H)Ank induce high levels of Nrarp expression, whereas XSu(H)DBM inhibits the expression of Nrarp. (B) Xenopus embryos at the two-cell stage were injected with RNA encoding Xenopus neurogenin (Ngn1) or various components of the Notch pathway, along with RNA encoding the neural inducer Noggin. (DBM) DNA-binding mutant of XSu(H); ICDΔC is depicted in Figure 6B. Neuralized ectoderm was removed from embryos at blastula stages and assayed at stage 16 (early neurulae) for the expression of Nrarp, ESR1, and HES6 RNA using an RNase protection assay (RPA) (Wettstein et al. 1997). Each sample was also assayed for _EF-1_α RNA expression as a loading control. Note that Nrarp expression is activated by Notch signaling induced using either activated forms of the receptor, XSu(H), or with ectopic ligand expression.
Figure 4
Nrarp overexpression blocks Notch signaling in embryos. Xenopus embryos at the two-cell stage were injected with RNA encoding Nrarp along with LacZ RNA as a tracer. At neural plate stages, embryos were fixed, stained for β-galactosidase expression, and double-labeled by whole-mount in situ hybridization for markers of neurons or ciliated cell precursors. (A) Nrarp overexpression induces the formation of additional primary neurons (arrow) as shown by the expression of a neural isoform of β-tubulin (N-Tub). Shown is dorsal view of the neural plate with the injected side oriented to the top of the panel. (B,C) Nrarp overexpression induces additional ciliated cell precursors that form in the skin as shown by the expression of an isoform of α-tubulin (α-Tub) (Deblandre et al. 1999). The uninjected and injected sides of the same embryo are shown in panels B and C, respectively. (D_–_F) Dorsal view of the neural plate with the injected side oriented up. Note that Nrarp overexpression induces more XDelta1 expression (D) but reduces the expression of ESR1 when scored at stage 12 or stage 14 (E and F, respectively).
Figure 5
Nrarp overexpression alters ICD-mediated transcription. (A) Xenopus embryos at the two-cell stage were injected with different concentrations of ICD RNA in either the presence or absence of RNA encoding Nrarp or Nrarptr. At blastula stage, animal caps were removed and cultured to the equivalent of stage 16, when they were assayed for expression of ESR1, ESR7, and _EF-1_α as described in Materials and Methods. Note that Nrarp overexpression reduced the levels of ESR1 and ESR7 expression induced by ICD. As a negative control, Nrarptr either had no effect or even slightly increased the levels of ESR gene expression, suggesting that it might have weak dominant-negative effects. Similarly, when ESR1 and ESR7 expression is induced by XDelta1, Nrarp overexpression reduces ESR expression, whereas Nrarptr slightly increases it. All assays included RNA encoding noggin to neuralize the ectoderm. (B) Time course experiments in which neuralized animal caps injected with the designated RNAs were extracted and assayed at the denoted developmental stages.
Figure 6
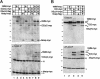
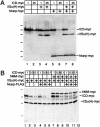
Nrarp forms a complex with XSu(H) and ICD. (A) RNA encoding myc-tagged forms of ICD and XSu(H) was injected along with Flag-tagged Nrarp into two-cell stage embryos. Extracts were prepared from animal tissue at blastula stages and subjected to Western analysis either directly (left; Extracts) or after coimmunoprecipitation (co-IP) with an α-Flag antibody (right; αFLAG IP) as described in the Materials and Methods. The myc-tagged proteins on Western blots were detected using the 9E10 α-myc monoclonal antibody, a secondary α-mouse IgG conjugated with horseradish peroxidase, and the ECL reagent. Note that neither ICD (lane 6) nor XSu(H) (lane 7) co-IP with Flag-tagged Nrarp unless both are present (lane 8). (H and L) Position of heavy and light chains, respectively, of the α-Flag antibody in this and subsequent figures. Similarly, the position of molecular weight markers is denoted here and elsewhere by a series of hatches left of the panel. (B) Diagram of the various forms of ICD and XSu(H) used for deletion analysis. Note that these deletions of ICD include a nuclear localization signal (NLS) that was added by cloning into the CS2-NLS vector (Turner and Weintraub 1994). (C) Co-IP of various myc-tagged ICD and XSu(H) with Nrarp-Flag. Shown is the Western analysis using the α-myc antibody of total extracts (top) or a co-IP with the α-Flag antibody (bottom). Note that both XSu(H)-myc and ICD-myc co-IP with Nrarp-Flag when all three are present (lane 5), but that this complex does not form with a truncated form of XSu(H) (lane 2). Moreover, both ICDΔC-myc (lane 6) and ICDAnk-myc (lane 8) form a complex with XSu(H)-myc and ICD-myc, but ICDram23-myc (lane 7) does not. Note that XSu(H)-myc and ICDΔC-myc comigrate at the same position on the gel. (D) Co-IP and Western analysis of embryo extracts expressing XSu(H)-Flag, as well as ICD-myc and Nrarp-myc. (Top) Western analysis of total extracts with the α-myc antibody; (bottom) same analysis of the proteins co-IPed with XSu(H)-Flag. Note that the amount of ICD associated with XSu(H) increases in the presence of Nrarp (cf. lanes 4 and 6). Similar results are obtained with ICDΔC (cf. lanes 7 and 8) and ICDAnk (cf. lanes 11 and 12) but not with ICDram23 (cf. lanes 9 and 10). Note also that Nrarp-myc co-IPs with XSu(H)-Flag in the presence of ICD-myc (lane 6), ICDΔC-myc (lane 8), and ICDAnk-myc (lane 12), but not ICDram23-myc (lane 10).
Figure 7
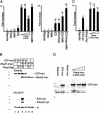
The Nrarp complex can include Mastermind. (A) Human Mastermind (hMM) forms a complex with XSu(H) and ICD. Embryo extracts expressing Flag-tagged XSu(H), as well as myc-tagged ICDΔC, Nrarp, and human Mastermind were subjected to co-IP and Western analysis using an α-myc antibody. (Top) Total extracts; (bottom) products that co-IP with an α-Flag antibody. Note that hMM binds to Su(H), but only in the presence of ICDΔC, and increases the levels of ICDΔC associated with XSu(H) (lane 8 vs. lane 6). Inclusion of Nrarp forms a complex that is co-IPed with XSu(H) (lane 9). (B) Co-IP and Western analysis of embryo extracts expressing Nrarp-Flag, XSu(H)-myc, ICD-myc, and hMM-myc. Note that hMM is included in a co-IP complex with ICD and XSu(H) that is recovered using Nrarp-Flag (lane 5, bottom). The low levels of ICD and hMM recovered in lane 4 presumably reflect the fact that XSu(H) is expressed ubiquitously and thus already present in extracts.
Figure 8
Nrarp reduces the levels of ICD in total cell extracts. (A) Two different concentrations of RNA encoding ICD-myc were injected into embryos alone or in the presence of XSu(H)-myc and Nrarp-myc. Shown is a Western analysis of the extracts prepared from these embryos using an α-myc antibody. Note that the presence of Nrarp decreases the levels of ICD-myc in the extracts (cf. lanes 6 and 2), and this decrease is greater in the presence of XSu(H) (cf. lanes 8 and 6). (B) Three different levels of ICD RNA were injected into embryos along with XSu(H)-myc in the presence and absence of Nrarp-Flag or hMM-myc. Shown is a Western blot with total extracts probed with a anti-myc antibody. Note that hMM-myc does not detectably change the levels of ICD-myc (lanes 7–9; see also Fig. 7A), nor does hMM-myc block the degradation promoted by Nrarp-Flag (lanes 10–12).
Figure 9
Nrarp potentiates Su(H)-dependent ICD activity in vitro. (A) HeLa cells were transfected transiently with a luciferase reporter containing multimerized Su(H) binding sites plus effectors. (Left) Activation of the reporter by ICD, ICD plus increasing levels of Nrarp, and ICD plus hMM. Cells were transfected with an ICD expression vector at a level resulting in submaximal (sixfold) activation. For details of transfection conditions see Materials and Methods. Cotransfection of Nrarp with ICD produced an approximately sevenfold increase in activity over ICD alone, an increase comparable to that seen with cotransfection of hMM with ICD. (Right) When ICD was transfected at levels producing higher activation of the reporter (30-fold), addition of Nrarp or hMM with ICD had little effect on activity. A combination of both Nrarp and hMM with ICD, however, produced an approximately threefold increase in activity over ICD alone. Bars represent means of triplicate measurements, and the standard error of the mean is shown. The activity of the reporter alone is defined arbitrarily as 1. Luciferase values are normalized to the activity of a cotransfected β-galactosidase vector. (B) Co-IP and Western analysis of 293T cells transfected with a tagged form of Nrarp (Nrarp-flag), as well as with myc-tagged forms of ICD and XSu(H). (Top) Western analysis of cell extracts with the α-myc antibody; (bottom) parallel analysis of proteins co-IPed with Nrarp-Flag. Note that ICD-myc or XSu(H)-myc coprecipitate with Nrarp only in a ternary complex (cf. lane 3 and lanes 2,5). (C) Reporter assay and (D) corresponding Western blot of Nrarp-myc cotransfected with ICD-myc in HeLa cells. In this experiment a modest potentiation of luciferase activity is observed when Nrarp-myc is cotransfected with high levels of ICD-myc, as is the case in the right panel of A. However, in HeLa cells no degradation of ICD-myc is observed in the presence of even the highest levels of Nrarp-myc.
Figure 10
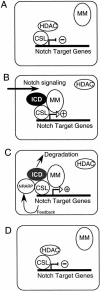
Model for Nrarp feedback regulation of Notch signaling. (A) In the absence of ICD, the CSL proteins associate with HDAC-containing complexes, thereby repressing transcription of Notch target genes. (B) On Notch signaling, ICD is released from the membrane and forms a complex with the CSL proteins and Mastermind (MM), thereby generating a transcriptional activator of Notch target genes, including Nrarp. (C) Nrarp feeds back and forms a complex with the CSL proteins, ICD, and Mastermind, which leads indirectly to a degradation of ICD. (D) Loss of ICD precludes the further association of Mastermind and Nrarp with the CSL proteins, thereby converting these proteins back into transcriptional repressors.
Similar articles
- The Nrarp gene encodes an ankyrin-repeat protein that is transcriptionally regulated by the notch signaling pathway.
Krebs LT, Deftos ML, Bevan MJ, Gridley T. Krebs LT, et al. Dev Biol. 2001 Oct 1;238(1):110-9. doi: 10.1006/dbio.2001.0408. Dev Biol. 2001. PMID: 11783997 - XSu(H)2 is an essential factor for gene expression and morphogenesis of the Xenopus gastrula embryo.
Ito M, Katada T, Miyatani S, Kinoshita T. Ito M, et al. Int J Dev Biol. 2007;51(1):27-36. doi: 10.1387/ijdb.062211mi. Int J Dev Biol. 2007. PMID: 17183462 - Direct regulation of the Nrarp gene promoter by the Notch signaling pathway.
Pirot P, van Grunsven LA, Marine JC, Huylebroeck D, Bellefroid EJ. Pirot P, et al. Biochem Biophys Res Commun. 2004 Sep 17;322(2):526-34. doi: 10.1016/j.bbrc.2004.07.157. Biochem Biophys Res Commun. 2004. PMID: 15325262 - HES and HERP families: multiple effectors of the Notch signaling pathway.
Iso T, Kedes L, Hamamori Y. Iso T, et al. J Cell Physiol. 2003 Mar;194(3):237-55. doi: 10.1002/jcp.10208. J Cell Physiol. 2003. PMID: 12548545 Review. - Notch signaling pathway.
Ehebauer M, Hayward P, Martinez-Arias A. Ehebauer M, et al. Sci STKE. 2006 Dec 5;2006(364):cm7. doi: 10.1126/stke.3642006cm7. Sci STKE. 2006. PMID: 17148788 Review.
Cited by
- Decoding breast cancer tissue-stroma interactions using species-specific sequencing.
Chivukula IV, Ramsköld D, Storvall H, Anderberg C, Jin S, Mamaeva V, Sahlgren C, Pietras K, Sandberg R, Lendahl U. Chivukula IV, et al. Breast Cancer Res. 2015 Aug 13;17(1):109. doi: 10.1186/s13058-015-0616-x. Breast Cancer Res. 2015. PMID: 26265142 Free PMC article. - MAML1: a coregulator that alters endometrial epithelial cell adhesive capacity.
Zafir S, Zhou W, Menkhorst E, Santos L, Dimitriadis E. Zafir S, et al. Fertil Res Pract. 2021 Mar 27;7(1):8. doi: 10.1186/s40738-021-00100-y. Fertil Res Pract. 2021. PMID: 33773601 Free PMC article. - Endothelial Notch signaling directly regulates the small GTPase RND1 to facilitate Notch suppression of endothelial migration.
Swaminathan B, Youn SW, Naiche LA, Du J, Villa SR, Metz JB, Feng H, Zhang C, Kopan R, Sims PA, Kitajewski JK. Swaminathan B, et al. Sci Rep. 2022 Jan 31;12(1):1655. doi: 10.1038/s41598-022-05666-1. Sci Rep. 2022. PMID: 35102202 Free PMC article. - Targetable NOTCH1 rearrangements in reninoma.
Treger TD, Lawrence JEG, Anderson ND, Coorens THH, Letunovska A, Abby E, Lee-Six H, Oliver TRW, Al-Saadi R, Tullus K, Morcrette G, Hutchinson JC, Rampling D, Sebire N, Pritchard-Jones K, Young MD, Mitchell TJ, Jones PH, Tran M, Behjati S, Chowdhury T. Treger TD, et al. Nat Commun. 2023 Sep 25;14(1):5826. doi: 10.1038/s41467-023-41118-8. Nat Commun. 2023. PMID: 37749094 Free PMC article. - Inhibition of myogenesis by Notch: evidence for multiple pathways.
Buas MF, Kabak S, Kadesch T. Buas MF, et al. J Cell Physiol. 2009 Jan;218(1):84-93. doi: 10.1002/jcp.21571. J Cell Physiol. 2009. PMID: 18727102 Free PMC article.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
- Beatus P, Lendahl U. Notch and neurogenesis. J Neurosci Res. 1998;54:125–136. - PubMed
- Bray S, Furriols M. Notch pathway: Making sense of Suppressor of Hairless. Curr Biol. 2001;11:R217–R221. - PubMed
- Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux R, Black A, Israel A. A novel proteolytic cleavage involved in Notch signaling: The role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. - PubMed
- Chitnis A, Kintner C. Neural induction and neurogenesis in amphibian embryos. Perspect Dev Neurobiol. 1995;3:3–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources