The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development - PubMed (original) (raw)
The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development
E Reissmann et al. Genes Dev. 2001.
Abstract
Nodal proteins have crucial roles in mesendoderm formation and left-right patterning during vertebrate development. The molecular mechanisms of signal transduction by Nodal and related ligands, however, are not fully understood. In this paper, we present biochemical and functional evidence that the orphan type I serine/threonine kinase receptor ALK7 acts as a receptor for mouse Nodal and Xenopus Nodal-related 1 (Xnr1). Receptor reconstitution experiments indicate that ALK7 collaborates with ActRIIB to confer responsiveness to Xnr1 and Nodal. Both receptors can independently bind Xnr1. In addition, Cripto, an extracellular protein genetically implicated in Nodal signaling, can independently interact with both Xnr1 and ALK7, and its expression greatly enhances the ability of ALK7 and ActRIIB to respond to Nodal ligands. The Activin receptor ALK4 is also able to mediate Nodal signaling but only in the presence of Cripto, with which it can also interact directly. A constitutively activated form of ALK7 mimics the mesendoderm-inducing activity of Xnr1 in Xenopus embryos, whereas a dominant-negative ALK7 specifically blocks the activities of Nodal and Xnr1 but has little effect on other related ligands. In contrast, a dominant-negative ALK4 blocks all mesoderm-inducing ligands tested, including Nodal, Xnr1, Xnr2, Xnr4, and Activin. In agreement with a role in Nodal signaling, ALK7 mRNA is localized to the ectodermal and organizer regions of Xenopus gastrula embryos and is expressed during early stages of mouse embryonic development. Therefore, our results indicate that both ALK4 and ALK7 can mediate signal transduction by Nodal proteins, although ALK7 appears to be a receptor more specifically dedicated to Nodal signaling.
Figures
Figure 1
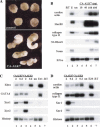
Functional characterization of ALK7 by gain-of-function experiments in Xenopus laevis. (A) Morphology of animal caps expressing the constitutively active mutant form of rat ALK7 (CA-ALK7). Embryos were injected in the animal pole at the two-cell stage, animal cap explants were dissected at the blastula stage and allowed to develop until sibling control embryos reached tailbud stage 25. (B) Dose-response induction of mesendodermal marker genes in animal caps injected with increasing concentrations of CA-ALK7. Embryos were injected at the two-cell stage, animal cap explants were dissected at the blastula stage, and harvested at stage 25. EF1α is shown as loading control. (−RT) No reverse transcriptase; (E) total embryo; (un) uninjected control explants. Data are representative of four independent experiments. (C) CA-ALK7 and CA-ALK4 induce early mesendodermal marker genes in a dose-responsive manner. RT–PCR analyses took place at the gastrula stage. (D) CA-ALK7 and CA-ALK4 both elicit late mesodermal marker genes, but show differences in their ability to maintain expression of Xnr genes in ectodermal explants. RT–PCR analyses were performed at the tailbud stage (stage 20).
Figure 2
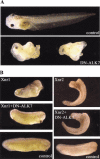
Role of ALK7 in cell fate and cell localization. (A–C) Control embryos injected with LacZ RNA. The β-gal staining at stage 45 is restricted to skin. (A) Dorsal view. (B) Ventral view. (C) Higher magnification of ventral view with focus on anterior embryonic regions. (D–G) Embryos were co-injected with CA-ALK7 mRNA and LacZ mRNA. The β-gal staining detects descendants of the injected ectodermal cells specifically localized to the gut, indicating an ectoderm-to-endoderm cell fate transformation and translocation. (D) High magnification with focus on the gut region. (E) Dorsal view. (F) Lateral view. (G) Ventral view.
Figure 3
Dominant-negative ALK7 (DN-ALK7) produces anterior defects and cyclopia, and inhibits Xnr1 activity in ectodermal explants and in whole embryos. (A) Embryonic phenotype of DN-ALK7 overexpression in vivo. Injection of 2 ng DN-ALK7 into all four marginal cells at the eight-cell stage resulted in anterior head defects, including cyclopia, and axial truncations (19% and 87% respectively, n = 46). Control sibling at late tadpole stage. Anterior to the left. (B) DN-ALK7 inhibits the formation of a partial secondary axis by Xnr1. Injection of 1 ng Xnr1 or Xnr2 RNA into one ventral-vegetal cell at the eight-cell stage resulted in a partial secondary axis (62%, total n = 32; 54%, total n = 22, respectively). Co-injection of 4 ng DN-ALK7 RNA inhibited the Xnr1-mediated phenotype (92%, total n = 28), whereas Xnr2 activity was unaffected. A sibling control embryo (stage 28) is shown in the bottom panels.
Figure 4
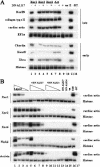
DN-ALK7 and DN-ALK4 inhibit mesoderm formation induced by Xnr1 and Nodal, whereas the activities of Xnr2, Xnr4, and Activin are only efficiently inhibited by DN-ALK4. (A) DN-ALK7 inhibits late and early mesodermal marker gene expression induced by Xnr1. DN-ALK7 alone did not induce any marker genes (lane 9). (un) Uninjected explants; (E) whole embryo control; (−RT) no reverse transcriptase. Stage 25 (late) and stage 11 (early) were analyzed. (B) Dose-response analysis of mesoderm-inducing ligand activity in the absence or presence of DN-ALK7 and DN-ALK4. (Lanes 1–4) Ligand (RNA concentrations for Xnr1, Xnr2, Xnr4, and Nodal were 1 ng, 0.5 ng, 0.1 ng, and 0.05 ng, respectively; RNA concentrations for Activin were 10 pg, 5 pg, 1 pg, and 0.5 pg, respectively); (lanes 5–8) ligands in the presence of 2 ng DN-ALK7; (lanes 9–12) ligands in the presence of 2 ng DN-ALK4; (lanes 13,14) DN-receptors alone.
Figure 5
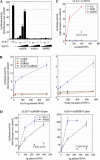
Reconstitution of functional receptors for Xnr1 and Nodal in heterologous cells. (A) Transactivation of ALK7 by overexpression of ActRIIB, but not ActRIIA or BMPRII, in HepG2 cells. The dose of CA− or wild-type (wt) ALK7 plasmid DNA was 1 ng per three wells. Type II receptors were transfected at 0.1, 0.3, 1, and 3 ng per three wells. (B) ALK7 and ActRIIB confer responsiveness to Xnr1 (left) and mouse Nodal (right) in heterologous cells. Ligand DNAs were transfected at the indicated amounts (per three wells). Results are expressed as mean ± SD, and are representative of three independent experiments. (C) ALK4 and ActRIIB do not suffice to confer responsiveness to Xnr1 and Nodal. HepG2 cells were transfected with ALK4, ActRIIB, and the indicated ligands. Results are expressed as mean ± SD, and are representative of three independent experiments. (D) Cripto enhances the response of ALK7 to Nodal proteins and allows Nodal and Xnr1 signaling via ALK4. HepG2 cells were transfected with receptors and ligands as indicated. In the absence of transfected ALK4 or ALK7 receptors, Cripto produced a much smaller response to Xnr1 and Nodal (Cripto alone), in agreement with the presence of low levels of endogenous Activin receptors in HepG2 cells. Results are expressed as mean ± SD, and are representative of three independent experiments.
Figure 6
ALK7, ActRIIB, and Cripto can bind Xnr1 directly, whereas ALK4 and ALK7 can associate with Cripto. (A) Binding of Xnr1 to soluble ALK7-Fc. Residual Activin signal in the ALK7-Fc lane is comparable with control, indicating background binding. (B) Binding of Xnr1 to soluble ActRIIB-Fc. As control, a purified GFRα1-Fc fusion was used (control Fc). (GFRα1 stands for glial cell line-derived neurotrophic factor (GDNF) family receptor α-1). (C) Cripto associates with ALK4 in the absence of ligand. Following precipitation with Protein-G beads, Cripto was detected by immunoblotting with anti-Histidine antibodies. Comparable amounts of Fc fusion proteins were used as determined by re-probing with anti-human IgG antibodies. (D) Reverse pull-down of ALK4-Fc and Cripto. Following precipitation with metal-affinity beads, ALK4-Fc was detected by immunoblotting with anti Fc antibodies. (E) Cripto associates with ALK7. Co-precipitation assay showing binding of purified ALK7-Fc to purified His-tagged Cripto. Following precipitation with metal-affinity beads, ALK7-Fc was detected by immunoblotting with anti-Fc antibodies. (F) Cripto binds Xnr1 directly. Co-precipitation assay showing binding of HA-tagged Xnr1 produced in COS cells to purified His-tagged Cripto. Following precipitation with metal-affinity beads, Xnr1 was detected by immunoblotting with anti-HA antibodies.
Figure 7
ALK7 mRNA is localized in the ectodermal and organizer regions of Xenopus gastrula embryos and is expressed during early stages of mouse embryonic development. (A) Amino acid sequence alignment of Xenopus and rat ALK7 fragments. The Xenopus ALK7 fragment obtained overlaps with the rat sequence from amino acid residues 259 to 312 located to the kinase domain. (+) Conservative changes; (−) non-conservative changes. (B) Phylogenetic analysis of homologous fragments from Xenopus and rat ALK7, and the related receptors ALK4 and ALK5. The tree was drawn using NJPLOT based on an alignment made in CLUSTALX. (C) Xenopus ALK7 is expressed maternally and persists throughout development as analyzed by RT–PCR on oocytes and embryos. (D) Expression of xALK7 is localized to dorsal-animal regions in early gastrula stage embryos. As control for correct embryo dissection the following marker genes were used: (Gsc) goosecoid (dorsal expression); (Xbra) Brachyury (dorsal–ventral or marginal); (msx) muscle-segment-homeobox-related gene (ventral); (EK) epidermal keratin (animal-ventral). Histone was used as loading control. (E) In situ hybridization analysis reveals expression of ALK7 in the organizer. Cross sections of those embryos show ALK7 expression in dorsal (d) and animal pole region (an). The blastopore lip (bp) is indicated. (v) Ventral; (veg) vegetal. (F) Expression of ALK7 mRNA in the early mouse embryo. RNA from the indicated days of mouse embryonic development (lanes 1–4), as well as adult brain mRNA (lane 5) and negative control (yeast tRNA, lane 6) were analyzed by RNase protection assay (RPA) for expression of ALK7 mRNA using a specific mouse ALK7 riboprobe.
Similar articles
- Cripto is a noncompetitive activin antagonist that forms analogous signaling complexes with activin and nodal.
Kelber JA, Shani G, Booker EC, Vale WW, Gray PC. Kelber JA, et al. J Biol Chem. 2008 Feb 22;283(8):4490-500. doi: 10.1074/jbc.M704960200. Epub 2007 Dec 18. J Biol Chem. 2008. PMID: 18089557 - ALK4 functions as a receptor for multiple TGF beta-related ligands to regulate left-right axis determination and mesoderm induction in Xenopus.
Chen Y, Mironova E, Whitaker LL, Edwards L, Yost HJ, Ramsdell AF. Chen Y, et al. Dev Biol. 2004 Apr 15;268(2):280-94. doi: 10.1016/j.ydbio.2003.12.035. Dev Biol. 2004. PMID: 15063168 - Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms.
Yeo C, Whitman M. Yeo C, et al. Mol Cell. 2001 May;7(5):949-57. doi: 10.1016/s1097-2765(01)00249-0. Mol Cell. 2001. PMID: 11389842 - Signal transduction pathway through activin receptors as a therapeutic target of musculoskeletal diseases and cancer.
Tsuchida K, Nakatani M, Uezumi A, Murakami T, Cui X. Tsuchida K, et al. Endocr J. 2008 Mar;55(1):11-21. doi: 10.1507/endocrj.kr-110. Epub 2007 Sep 14. Endocr J. 2008. PMID: 17878607 Review. - Cripto-1: a multifunctional modulator during embryogenesis and oncogenesis.
Strizzi L, Bianco C, Normanno N, Salomon D. Strizzi L, et al. Oncogene. 2005 Aug 29;24(37):5731-41. doi: 10.1038/sj.onc.1208918. Oncogene. 2005. PMID: 16123806 Review.
Cited by
- The pattern of nodal morphogen signaling is shaped by co-receptor expression.
Lord ND, Carte AN, Abitua PB, Schier AF. Lord ND, et al. Elife. 2021 May 26;10:e54894. doi: 10.7554/eLife.54894. Elife. 2021. PMID: 34036935 Free PMC article. - A poised chromatin platform for TGF-β access to master regulators.
Xi Q, Wang Z, Zaromytidou AI, Zhang XH, Chow-Tsang LF, Liu JX, Kim H, Barlas A, Manova-Todorova K, Kaartinen V, Studer L, Mark W, Patel DJ, Massagué J. Xi Q, et al. Cell. 2011 Dec 23;147(7):1511-24. doi: 10.1016/j.cell.2011.11.032. Cell. 2011. PMID: 22196728 Free PMC article. - Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis.
Andersson O, Reissmann E, Ibáñez CF. Andersson O, et al. EMBO Rep. 2006 Aug;7(8):831-7. doi: 10.1038/sj.embor.7400752. Epub 2006 Jul 14. EMBO Rep. 2006. PMID: 16845371 Free PMC article. - Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning.
Oh SP, Yeo CY, Lee Y, Schrewe H, Whitman M, Li E. Oh SP, et al. Genes Dev. 2002 Nov 1;16(21):2749-54. doi: 10.1101/gad.1021802. Genes Dev. 2002. PMID: 12414726 Free PMC article. - Nodal and activin receptor-like kinase 7 induce apoptosis in human breast cancer cell lines: Role of caspase 3.
Zhong Y, Xu G, Ye G, Lee D, Modica-Amore J, Peng C. Zhong Y, et al. Int J Physiol Pathophysiol Pharmacol. 2009 Feb 27;1(1):83-96. Int J Physiol Pathophysiol Pharmacol. 2009. PMID: 21383881 Free PMC article.
References
- Armes A, Smith JC. The ALK-2 and ALK-4 Activin receptors transduce distinct mesoderm-inducing signals during early Xenopus development but do not co-operate to establish thresholds. Development. 1997;124:3797–3804. - PubMed
- Capdevila J, Vogan KJ, Tabin CJ, Izpisua Belmonte JC. Mechanisms of left–right determination in vertebrates. Cell. 2000;101:9–21. - PubMed
- Chang C, Hemmati-Brivanlou A. Xenopus GDF6, a new antagonist of noggin and a partner of BMPs. Development. 1999;126:3347–3357. - PubMed
- Chang C, Wilson PA, Mathews LS, Hemmati-Brivanlou A. A Xenopus type I receptor mediates mesodermal but not neural specification during embryogenesis. Development. 1997;124:827–837. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources