Parallel and independent regulation of interleukin-3 mRNA turnover by phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase - PubMed (original) (raw)
Parallel and independent regulation of interleukin-3 mRNA turnover by phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase
X F Ming et al. Mol Cell Biol. 2001 Sep.
Abstract
AU-rich elements (ARE) present in the 3' untranslated regions of many cytokines and immediate-early genes are responsible for targeting the transcripts for rapid decay. We present evidence from cotransfection experiments in NIH 3T3 cells that two signaling pathways, one involving phosphatidylinositol 3-kinase (PI3-K), and one involving the p38 mitogen-activated protein kinase (MAPK), lead to stabilization of interleukin-3 mRNA in parallel. Stabilization mediated by either of the two pathways was antagonized by tristetraprolin (TTP), an AU-binding protein known to promote constitutive decay of ARE-containing transcripts. Remarkably, the stabilizing AU-binding protein HuR, in collaboration with p38 MAPK but not with PI3-K, could overcome the destabilizing effect of TTP. These data argue that the stabilizing kinases PI3-K and p38 MAPK do not act through direct inactivation of TTP but via activating pathway-specific stabilizing AU-binding proteins. Our data suggest an integrated model of mRNA turnover control, where stabilizing (HuR) and destabilizing (TTP) AU-binding proteins compete and where the former are under the positive control of independent phosphokinase signaling pathways.
Figures
FIG. 1
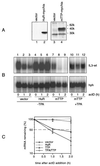
Stability of IL-3 transcripts in a transient-transfection assay. (A) NIH 3T3 B2A2 cells were cotransfected with 3 μg of Mxh-IL-3-wt and 1 μg of Mx-IL-3-ΔAU, the latter plasmid carrying a deletion of the ARE. hph served to monitor transfection efficiency and control for loading. Forty-eight hours after transfection, actD (5 μg/ml) was added for the indicated time in the absence (control; lanes 1 to 4) or presence of 2 μM ionomycin (iono; lanes 5 to 8) or 20 ng of TPA/ml (lanes 9 to 12). (B) Quantification of the signal intensities shown in panel A by PhosphorImager, with the hph-normalized values at time zero taken as 100%. Each point represents the average of three transfection experiments. (C) In vitro kinase assays. Cells were either untreated (con.; lane 1) or stimulated with 2 μM ionomycin (lane 2) or 20 ng of TPA/ml (lane 3) for 20 min. Then lysates were prepared, and 100 μg was subjected to in vitro JNK (a) or p38 MAPK (b) assay using either GST-jun (1–79) or GST-ATF2 (1–254), respectively, as the substrate.
FIG. 1
Stability of IL-3 transcripts in a transient-transfection assay. (A) NIH 3T3 B2A2 cells were cotransfected with 3 μg of Mxh-IL-3-wt and 1 μg of Mx-IL-3-ΔAU, the latter plasmid carrying a deletion of the ARE. hph served to monitor transfection efficiency and control for loading. Forty-eight hours after transfection, actD (5 μg/ml) was added for the indicated time in the absence (control; lanes 1 to 4) or presence of 2 μM ionomycin (iono; lanes 5 to 8) or 20 ng of TPA/ml (lanes 9 to 12). (B) Quantification of the signal intensities shown in panel A by PhosphorImager, with the hph-normalized values at time zero taken as 100%. Each point represents the average of three transfection experiments. (C) In vitro kinase assays. Cells were either untreated (con.; lane 1) or stimulated with 2 μM ionomycin (lane 2) or 20 ng of TPA/ml (lane 3) for 20 min. Then lysates were prepared, and 100 μg was subjected to in vitro JNK (a) or p38 MAPK (b) assay using either GST-jun (1–79) or GST-ATF2 (1–254), respectively, as the substrate.
FIG. 1
Stability of IL-3 transcripts in a transient-transfection assay. (A) NIH 3T3 B2A2 cells were cotransfected with 3 μg of Mxh-IL-3-wt and 1 μg of Mx-IL-3-ΔAU, the latter plasmid carrying a deletion of the ARE. hph served to monitor transfection efficiency and control for loading. Forty-eight hours after transfection, actD (5 μg/ml) was added for the indicated time in the absence (control; lanes 1 to 4) or presence of 2 μM ionomycin (iono; lanes 5 to 8) or 20 ng of TPA/ml (lanes 9 to 12). (B) Quantification of the signal intensities shown in panel A by PhosphorImager, with the hph-normalized values at time zero taken as 100%. Each point represents the average of three transfection experiments. (C) In vitro kinase assays. Cells were either untreated (con.; lane 1) or stimulated with 2 μM ionomycin (lane 2) or 20 ng of TPA/ml (lane 3) for 20 min. Then lysates were prepared, and 100 μg was subjected to in vitro JNK (a) or p38 MAPK (b) assay using either GST-jun (1–79) or GST-ATF2 (1–254), respectively, as the substrate.
FIG. 2
PI3-K and p38 MAPK are independently involved in IL-3 mRNA stabilization. (A) Effect of rCD2-p110, MKK7D, or MEK6DD on wt IL-3 mRNA decay. Three micrograms of the reporter plasmid Mxh-IL-3-wt was cotransfected with 2 μg of vector (lanes 1 to 3), rCD2-p110 (lanes 4 to 6), or MKK7D (lanes 7 to 9) or 0.5 μg of MEK6DD (lanes 10 to 12). Decay assays were performed as described in the legend to Fig. 1. Shown is a representative result from three independent experiments. For quantification, the averages of three transfection experiments are plotted in graph b. (B) Stimulation of JNK or p38 activity by MKK7, PI3-K, or MEK6. Cells were cotransfected with 2 μg of either M2-JNK (a and b) or M2-p38 (c and d) in combination with 2 μg of vector (lane 1), MKK7D (lane 2), rCD2-p110 (lane 3), or MEK6DD (lane 4). One hundred micrograms of the lysates was used for immunoprecipitation with the M2 monoclonal antibody and subjected to in vitro JNK (a) or p38 kinase (c) assay using GST–c-jun (1–79) or GST-ATF2 (1–254), respectively, as the substrate. The expression of M2-JNK (b) and M2-p38 (d) was analyzed by Western blotting using anti-M2. (C and D) Effect of JNK-APF or p38-AGF on MEK6DD- or rCD2-p110-mediated stabilization. Mxh-IL-3-wt reporter plasmid was transfected alone (D, panel a, lanes 1 to 3) or in combination with MEK6DD (C, panel a, lanes 1 to 12) or rCD2-p110 (D, panel a, lanes 4 to 12) in the absence (C, panel a, lanes 1 to 3; D, panel a, lanes 4 to 6) or presence of JNK-APF (C, panel a, lanes 4 to 6; D, panel a, lanes 7 to 9), 4 μg of p38-AGF (C, panel a, lanes 7 to 9; D, panel a, lanes 10 to 12), or 1 μg of p38-AGF (C, panel a, lanes 10 to 12). A decay assay was performed 2 days after transfection as described for panel A. Graph b shows quantification of signal intensities using the average of three transfection experiments. (C) Panel c reveals the expression of MEK6DD by Western blot analysis using the anti-hemagglutinin monoclonal antibody 12CA5.
FIG. 2
PI3-K and p38 MAPK are independently involved in IL-3 mRNA stabilization. (A) Effect of rCD2-p110, MKK7D, or MEK6DD on wt IL-3 mRNA decay. Three micrograms of the reporter plasmid Mxh-IL-3-wt was cotransfected with 2 μg of vector (lanes 1 to 3), rCD2-p110 (lanes 4 to 6), or MKK7D (lanes 7 to 9) or 0.5 μg of MEK6DD (lanes 10 to 12). Decay assays were performed as described in the legend to Fig. 1. Shown is a representative result from three independent experiments. For quantification, the averages of three transfection experiments are plotted in graph b. (B) Stimulation of JNK or p38 activity by MKK7, PI3-K, or MEK6. Cells were cotransfected with 2 μg of either M2-JNK (a and b) or M2-p38 (c and d) in combination with 2 μg of vector (lane 1), MKK7D (lane 2), rCD2-p110 (lane 3), or MEK6DD (lane 4). One hundred micrograms of the lysates was used for immunoprecipitation with the M2 monoclonal antibody and subjected to in vitro JNK (a) or p38 kinase (c) assay using GST–c-jun (1–79) or GST-ATF2 (1–254), respectively, as the substrate. The expression of M2-JNK (b) and M2-p38 (d) was analyzed by Western blotting using anti-M2. (C and D) Effect of JNK-APF or p38-AGF on MEK6DD- or rCD2-p110-mediated stabilization. Mxh-IL-3-wt reporter plasmid was transfected alone (D, panel a, lanes 1 to 3) or in combination with MEK6DD (C, panel a, lanes 1 to 12) or rCD2-p110 (D, panel a, lanes 4 to 12) in the absence (C, panel a, lanes 1 to 3; D, panel a, lanes 4 to 6) or presence of JNK-APF (C, panel a, lanes 4 to 6; D, panel a, lanes 7 to 9), 4 μg of p38-AGF (C, panel a, lanes 7 to 9; D, panel a, lanes 10 to 12), or 1 μg of p38-AGF (C, panel a, lanes 10 to 12). A decay assay was performed 2 days after transfection as described for panel A. Graph b shows quantification of signal intensities using the average of three transfection experiments. (C) Panel c reveals the expression of MEK6DD by Western blot analysis using the anti-hemagglutinin monoclonal antibody 12CA5.
FIG. 3
Role of HuR and TTP in IL-3 mRNA turnover in vivo. (A) Expression of myc-tagged HuR (lane 2) and TTP (lane 4) detected by Western blotting using monoclonal anti-myc antibody 9E10. (B) Cells were transfected with Mxh-IL-3-wt reporter plasmid alone (lanes 1 to 3) or with 1 μg of HuR (lanes 4 to 6) or 0.3 μg of TTP (lanes 7 to 12). The effect of the ectopically expressed AUBPs on constitutive IL-3 mRNA decay (lanes 1 to 9) or on TPA-induced stabilization (lanes 10 to 12) was assessed in a decay assay. Note that 2 μg of TTP was transfected for the Western blot analysis shown in panel A, whereas only 0.3 μg was used in all other decay assays, since 2 μg of TTP decreased the IL-3 mRNA signal to undetectable levels while TTP was not detectable by Western blotting when only 0.3 μg was transfected (data not shown). (C) Quantification of signal intensities, using the average of three transfection experiments.
FIG. 4
Mutual interactions of upstream kinases and AUBPs in regulating IL-3 mRNA turnover. (A) Effect of TTP on PI3-K-, p38 MAPK-, or HuR-mediated stabilization. Cells were transfected with Mxh-IL-3-wt reporter plasmid alone (a and b, lanes 1 to 3) or together with active kinases (a, lanes 4 to 18) or stabilizing HuR (b, lanes 4 to 6 and 10 to 12) in the absence (a, lanes 1 to 6 and 13 to 15; b, lanes 1 to 6) or presence of 0.3 μg of TTP-wt (a, lanes 7 to 9 and 16 to 18; b, lanes 7 to 12) or 1 μg of TTP-C139R (a, lanes 10 to 12). Shown in graphs c are the averages of three transfection experiments. (B) Effect of TTP (lanes 7 to 9) on stabilization mediated by a combination of rCD2-p110 with MEK6DD (lanes 4 to 9). The experiment was performed as described for panel A, and the plasmids were transfected as indicated in panel a. The averages of three transfection experiments are shown in graph b. (C and D) mRNA decay assay was performed using cells transfected with Mxh-IL-3-wt alone (C, panel a, lanes 1 to 3) or in combination with MEK6DD (C, panel a, lanes 4 to 12) or rCD2-p110 (D, panel a, lanes 1 to 9) in the absence (C, panel a, lanes 4 to 9; D, panel a, lanes 1 to 6) or presence of HuR (C, panel a, lanes 10 to 12; D, panel a, lanes 7 to 9). In addition, TTP was cotransfected in lanes 7 to 12 (C, panel a) or lanes 4 to 9 (D, panel a). Shown is one representative result from three transfection experiments. Quantification of the signal intensities using the average of three transfection experiments is shown in graph b. Panels c show the expression of MEK6DD and HuR (C) or rCD2-p110 and HuR (D) by Western blotting. rCD2-p110 expression was detected with anti-rCD2 antibody.
FIG. 4
Mutual interactions of upstream kinases and AUBPs in regulating IL-3 mRNA turnover. (A) Effect of TTP on PI3-K-, p38 MAPK-, or HuR-mediated stabilization. Cells were transfected with Mxh-IL-3-wt reporter plasmid alone (a and b, lanes 1 to 3) or together with active kinases (a, lanes 4 to 18) or stabilizing HuR (b, lanes 4 to 6 and 10 to 12) in the absence (a, lanes 1 to 6 and 13 to 15; b, lanes 1 to 6) or presence of 0.3 μg of TTP-wt (a, lanes 7 to 9 and 16 to 18; b, lanes 7 to 12) or 1 μg of TTP-C139R (a, lanes 10 to 12). Shown in graphs c are the averages of three transfection experiments. (B) Effect of TTP (lanes 7 to 9) on stabilization mediated by a combination of rCD2-p110 with MEK6DD (lanes 4 to 9). The experiment was performed as described for panel A, and the plasmids were transfected as indicated in panel a. The averages of three transfection experiments are shown in graph b. (C and D) mRNA decay assay was performed using cells transfected with Mxh-IL-3-wt alone (C, panel a, lanes 1 to 3) or in combination with MEK6DD (C, panel a, lanes 4 to 12) or rCD2-p110 (D, panel a, lanes 1 to 9) in the absence (C, panel a, lanes 4 to 9; D, panel a, lanes 1 to 6) or presence of HuR (C, panel a, lanes 10 to 12; D, panel a, lanes 7 to 9). In addition, TTP was cotransfected in lanes 7 to 12 (C, panel a) or lanes 4 to 9 (D, panel a). Shown is one representative result from three transfection experiments. Quantification of the signal intensities using the average of three transfection experiments is shown in graph b. Panels c show the expression of MEK6DD and HuR (C) or rCD2-p110 and HuR (D) by Western blotting. rCD2-p110 expression was detected with anti-rCD2 antibody.
FIG. 5
ARE is the cis element through which IL-3 mRNA turnover is regulated by PI3-K or p38 MAPK involving AUBPs. (A) Effect of TTP on the decay of β-globin reporter transcripts. Cells were transfected with Mxh-β-IL3(UTR)wt (lanes 1 to 6) or Mxh-β-IL3(UTR)ΔAU (lanes 7 to 12) in the absence (lanes 1 to 3 and 7 to 9) or presence (lanes 4 to 6 and 10 to 12) of 0.3 μg of TTP. (B) The experiment was performed as described in the legend to Fig. 4C except that Mxh-β-IL3(UTR)wt was used as the reporter construct instead of Mxh-IL-3-wt. Shown in panel A, graph b, and panel B, graph c are the average values taken from three transfection experiments.
FIG. 5
ARE is the cis element through which IL-3 mRNA turnover is regulated by PI3-K or p38 MAPK involving AUBPs. (A) Effect of TTP on the decay of β-globin reporter transcripts. Cells were transfected with Mxh-β-IL3(UTR)wt (lanes 1 to 6) or Mxh-β-IL3(UTR)ΔAU (lanes 7 to 12) in the absence (lanes 1 to 3 and 7 to 9) or presence (lanes 4 to 6 and 10 to 12) of 0.3 μg of TTP. (B) The experiment was performed as described in the legend to Fig. 4C except that Mxh-β-IL3(UTR)wt was used as the reporter construct instead of Mxh-IL-3-wt. Shown in panel A, graph b, and panel B, graph c are the average values taken from three transfection experiments.
FIG. 6
Integrated model of ARE-mediated mRNA turnover control. IL-3 mRNA turnover is under the control of both stabilizing (HuR) and destabilizing (TTP) AUBPs. Under resting conditions, the destabilizing function of TTP is dominant and responsible for constitutive rapid decay of the transcripts. Upon stimulation, independent signaling pathways become activated and lead either to activation of the stabilizing AUBPs or inactivation of destabilizing AUBPs, as a result of which degradation of the transcripts is blocked. The question marks indicate that the element is hypothetical.
Similar articles
- Cellular mutants define a common mRNA degradation pathway targeting cytokine AU-rich elements.
Stoecklin G, Stoeckle P, Lu M, Muehlemann O, Moroni C. Stoecklin G, et al. RNA. 2001 Nov;7(11):1578-88. RNA. 2001. PMID: 11720287 Free PMC article. - Structural and functional dissection of a conserved destabilizing element of cyclo-oxygenase-2 mRNA: evidence against the involvement of AUF-1 [AU-rich element/poly(U)-binding/degradation factor-1], AUF-2, tristetraprolin, HuR (Hu antigen R) or FBP1 (far-upstream-sequence-element-binding protein 1).
Sully G, Dean JL, Wait R, Rawlinson L, Santalucia T, Saklatvala J, Clark AR. Sully G, et al. Biochem J. 2004 Feb 1;377(Pt 3):629-39. doi: 10.1042/BJ20031484. Biochem J. 2004. PMID: 14594446 Free PMC article. - Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability.
Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J, Clark AR. Mahtani KR, et al. Mol Cell Biol. 2001 Oct;21(19):6461-9. doi: 10.1128/MCB.21.9.6461-6469.2001. Mol Cell Biol. 2001. PMID: 11533235 Free PMC article. - The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation.
Dean JL, Sully G, Clark AR, Saklatvala J. Dean JL, et al. Cell Signal. 2004 Oct;16(10):1113-21. doi: 10.1016/j.cellsig.2004.04.006. Cell Signal. 2004. PMID: 15240006 Review. - Is MK2 (mitogen-activated protein kinase-activated protein kinase 2) the key for understanding post-transcriptional regulation of gene expression?
Kotlyarov A, Gaestel M. Kotlyarov A, et al. Biochem Soc Trans. 2002 Nov;30(Pt 6):959-63. doi: 10.1042/bst0300959. Biochem Soc Trans. 2002. PMID: 12440954 Review.
Cited by
- Decreased sensitivity of tristetraprolin-deficient cells to p38 inhibitors suggests the involvement of tristetraprolin in the p38 signaling pathway.
Carballo E, Cao H, Lai WS, Kennington EA, Campbell D, Blackshear PJ. Carballo E, et al. J Biol Chem. 2001 Nov 9;276(45):42580-7. doi: 10.1074/jbc.M104953200. Epub 2001 Sep 6. J Biol Chem. 2001. PMID: 11546803 Free PMC article. - Akt2, a novel functional link between p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in myogenesis.
Gonzalez I, Tripathi G, Carter EJ, Cobb LJ, Salih DA, Lovett FA, Holding C, Pell JM. Gonzalez I, et al. Mol Cell Biol. 2004 May;24(9):3607-22. doi: 10.1128/MCB.24.9.3607-3622.2004. Mol Cell Biol. 2004. PMID: 15082758 Free PMC article. - Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: implications for cellular senescence.
Abdelmohsen K, Kuwano Y, Kim HH, Gorospe M. Abdelmohsen K, et al. Biol Chem. 2008 Mar;389(3):243-55. doi: 10.1515/BC.2008.022. Biol Chem. 2008. PMID: 18177264 Free PMC article. Review. - Coordinate regulation of GATA-3 and Th2 cytokine gene expression by the RNA-binding protein HuR.
Stellato C, Gubin MM, Magee JD, Fang X, Fan J, Tartar DM, Chen J, Dahm GM, Calaluce R, Mori F, Jackson GA, Casolaro V, Franklin CL, Atasoy U. Stellato C, et al. J Immunol. 2011 Jul 1;187(1):441-9. doi: 10.4049/jimmunol.1001881. Epub 2011 May 25. J Immunol. 2011. PMID: 21613615 Free PMC article. - Posttranscriptional Gene Regulation: Novel Pathways for Glucocorticoids' Anti-inflammatory Action.
Stellato C. Stellato C. Transl Med UniSa. 2012 Apr 30;3:67-73. Print 2012 May. Transl Med UniSa. 2012. PMID: 23905055 Free PMC article.
References
- Carballo E, Lai W S, Blackshear P J. Feedback inhibition of macrophage tumor necrosis factor-α production by tristetraprolin. Science. 1998;281:1001–1005. - PubMed
- Chen C-Y, Gatto-Konczak F D, Wu Z, Karin M. Stabilization of interleukin-2 mRNA by the c-jun NH2-terminal kinase pathway. Science. 1998;280:1945–1949. - PubMed
- Chen C-Y A, Shyu A-B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous