Loss of HuR is linked to reduced expression of proliferative genes during replicative senescence - PubMed (original) (raw)
Loss of HuR is linked to reduced expression of proliferative genes during replicative senescence
W Wang et al. Mol Cell Biol. 2001 Sep.
Abstract
Cellular aging is accompanied by alterations in gene expression patterns. Here, using two models of replicative senescence, we describe the influence of the RNA-binding protein HuR in regulating the expression of several genes whose expression decreases during senescence. We demonstrate that HuR levels, HuR binding to target mRNAs encoding proliferative genes, and the half-lives of such mRNAs are lower in senescent cells. Importantly, overexpression of HuR in senescent cells restored a "younger" phenotype, while a reduction in HuR expression accentuated the senescent phenotype. Our studies highlight a critical role for HuR during the process of replicative senescence.
Figures
FIG. 1
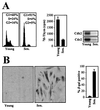
Phenotypic characterization of IDH4 cells cultured in the presence or absence of dex. IDH4 cells that were cultured in the presence (Young) or absence (Sen_._) of dex for 7 days were subjected to FACS analysis, 3H-thymidine incorporation assays, and assessment of cdk2- and cdc2-associated kinase activity using histone H1 as a substrate (A) and to SA-β-gal staining (B) (left, representative fields; right, quantitation of SA-β-gal-positive IDH4 cells). The graphs represent the means + standard errors of the means of four independent experiments.
FIG. 2
Senescence-associated gene expression in WI-38 and IDH4 fibroblasts. (A) Northern blot analysis of expression of the genes indicated using early-passage (Young; ≈28 pdl) and late-passage (senescent [Sen.]; ≈60 pdl) WI-38 cells, as well as young (dex-treated) and senescent (7 days after removing dex) IDH4 cells. (B) Stabilities of cyclin A, cyclin B1, c-fos, and β-actin mRNAs in IDH4 cells that were either dex-treated [Young (+dex)] or cultured without dex for 7 days [Senescent (−dex)] were assessed after the addition of 2 μg of actinomycin D/ml; preparation of RNA at the times indicated; measurement of cyclin A, cyclin B1, c-fos, and β-actin mRNA Northern blot signals; normalizing them to 18S rRNA; and plotting them on a logarithmic scale (bottom). Dashed horizontal lines, 50% of untreated. The data represent the means ± standard errors of the means of four independent experiments.
FIG. 3
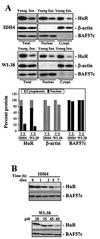
HuR expression in young and senescent human fibroblasts. (A) Western blot analysis of HuR expression in whole-cell (Total [20 μg]), nuclear (10 μg), or cytoplasmic (Cytopl. [40 μg]) lysates from either WI-38 or IDH4 populations (young or senescent [Sen.], as described in the legend to Fig. 1A). Western blot analysis of BAF57c and β-actin expression served to assess the quality of the cell fractionation procedure and to monitor differences in loading and transfer among samples. Western blot signals were quantitated and represented (graph) relative to the total protein present in whole-cell lysates from young cells. Y, young; S, senescent. (B) Western blot analyses depicting time-dependent changes in total HuR expression in dex-depleted (−Dex) IDH4 cells or in WI-38 cells of the indicated number of population doublings.
FIG. 4
HuR expression in fibroblasts from skin biopsies from young and elderly individuals. Western blot analysis of expression of the proteins indicated was carried out using fibroblasts from skin biopsies obtained from individual donors who were either young (15 to 30 years old) or aged (80 to 94 years old). The fibroblasts were cultured in vitro for 3 to 4 pdl before Western blot analysis of whole-cell lysates. The error bars represent standard errors of the mean.
FIG. 5
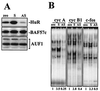
HuR binding to target mRNAs in young and senescent human fibroblasts. Radiolabeled RNAs encoding the 3′ UTRs of c-fos, cyclin A, cyclin B1, and cyclin E are shown (top; underlined). Radiolabeled transcripts were incubated with proteins present in either nuclear (Nuc_._) or cytoplasmic lysates of young (Y) or senescent (S) WI-38 or IDH4 cells (defined in the legend to Fig. 3), forming associations with slower electrophoretic mobilities (bottom). Complexes formed with lysates from IDH4 cells and radiolabeled transcripts were assayed for the ability to be supershifted by either anti-HuR antibodies (α_-_HuR), or control anti-p27, anti-p38, or anti-p53 antibodies (α_-_p27, α_-_p38, and α_-_p53, respectively), as shown. f, free probes; arrows, supershifted complexes.
FIG. 6
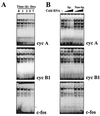
Time course of complex formation in cells undergoing senescence, showing specificity of binding to target radiolabeled transcripts. (A) Cytoplasmic lysates from IDH4 cells cultured without dex (−Dex) for the indicated times were incubated with radiolabeled cyclin A, cyclin B1, or c-fos transcripts. (B) IDH4 cytoplasmic lysates were incubated with 5×, 10×, or 20× excess unlabeled competitor transcripts. Lanes Sp, competition using a specific transcript (unlabeled cyclin [cyc] A RNA competing for binding of radiolabeled cyclin A RNA, unlabeled cyclin B1 RNA competing for binding of radiolabeled cyclin B1 RNA, and likewise for c-fos RNA); lanes Non-Sp, competition using unlabeled cyclin E RNA as a nonspecific transcript in binding assays with radiolabeled cyclin A RNA, cyclin B1 RNA, and c-fos RNA.
FIG. 7
Influence of HuR levels on the formation of protein complexes with the 3′ UTRs of senescence-associated genes. (A) Western blot analysis of expression of the indicated genes was carried out using IDH4 cells transiently transfected with either empty vector (zeo), HuR-expressing (S), or antisense-HuR-expressing (AS) plasmid pZeoSV2(−) and then cultured in the absence of dex for 3 days. (B) Binding of radiolabeled RNAs corresponding to the 3′ UTRs of cyclin (cyc) A, cyclin B1, and c-fos and proteins present in the cytoplasmic lysates (10 μg) of IDH4 cells cultured as described for panel A. Fold differences in intensity of shifted complexes, relative to those in zeo populations, are shown below the lanes.
FIG. 8
Influence of HuR levels on the steady-state levels and stabilities of senescence-associated genes. Three days after transfection and removal of dex (as described in the legend to Fig. 7), expression of cyclin A, cyclin B1, and c-fos was assessed in IDH4 cells. (A) Representative Northern blots depicting the steady-state levels of cyclin (cyc) A, cyclin B1, c-fos, gadd153, and β-actin mRNAs, as well as 18S rRNA, and quantitations from 10 independent experiments (bar graph, showing means + standard errors of the means [SEM]). (B) Cyclin A, cyclin B1, β-actin, and gadd153 mRNA stabilities, assessed as explained in the legend to Fig. 2B; the values represent the means ± SEM of seven independent experiments.
FIG. 9
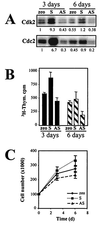
Influence of HuR levels on the senescent phenotype. IDH4 cells transfected as described in the legend to Fig. 7 (zeo, S, and AS) were cultured without dex for either 3 or 6 days and then subjected to assessment and quantitation of cdk2- and cdc2-associated kinase activity using histone H1 as a substrate (A), 3H-thymidine (Thym.) incorporation (B), or total cell numbers (C). The graphs represent the means ± standard errors of the means of four independent experiments.
FIG. 10
Influence of HuR levels on SA-β-gal activity. IDH4 cells transfected as described in the legend to Fig. 7 (zeo, S, and AS) were cultured without dex for 4 days and then stained to assess SA-β-gal activity. Top, representative fields; bottom, quantitation of SA-β-gal-positive IDH4 cells. The graph represents the means + standard errors of the means of four independent experiments.
Similar articles
- Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function.
Wang W, Yang X, López de Silanes I, Carling D, Gorospe M. Wang W, et al. J Biol Chem. 2003 Jul 18;278(29):27016-23. doi: 10.1074/jbc.M300318200. Epub 2003 May 1. J Biol Chem. 2003. PMID: 12730239 - Reduced nuclear export of HuR mRNA by HuR is linked to the loss of HuR in replicative senescence.
Yi J, Chang N, Liu X, Guo G, Xue L, Tong T, Gorospe M, Wang W. Yi J, et al. Nucleic Acids Res. 2010 Mar;38(5):1547-58. doi: 10.1093/nar/gkp1114. Epub 2009 Dec 8. Nucleic Acids Res. 2010. PMID: 20007147 Free PMC article. - Loss of CARM1 is linked to reduced HuR function in replicative senescence.
Pang L, Tian H, Chang N, Yi J, Xue L, Jiang B, Gorospe M, Zhang X, Wang W. Pang L, et al. BMC Mol Biol. 2013 Jul 9;14:15. doi: 10.1186/1471-2199-14-15. BMC Mol Biol. 2013. PMID: 23837869 Free PMC article. - Posttranscriptional orchestration of an anti-apoptotic program by HuR.
Abdelmohsen K, Lal A, Kim HH, Gorospe M. Abdelmohsen K, et al. Cell Cycle. 2007 Jun 1;6(11):1288-92. doi: 10.4161/cc.6.11.4299. Epub 2007 Jun 15. Cell Cycle. 2007. PMID: 17534146 Review. - HuR, a key post-transcriptional regulator, and its implication in progression of breast cancer.
Yuan Z, Sanders AJ, Ye L, Jiang WG. Yuan Z, et al. Histol Histopathol. 2010 Oct;25(10):1331-40. doi: 10.14670/HH-25.1331. Histol Histopathol. 2010. PMID: 20712017 Review.
Cited by
- The tRNA methyltransferase NSun2 stabilizes p16INK⁴ mRNA by methylating the 3'-untranslated region of p16.
Zhang X, Liu Z, Yi J, Tang H, Xing J, Yu M, Tong T, Shang Y, Gorospe M, Wang W. Zhang X, et al. Nat Commun. 2012 Mar 6;3:712. doi: 10.1038/ncomms1692. Nat Commun. 2012. PMID: 22395603 Free PMC article. - The mRNA decay factor tristetraprolin (TTP) induces senescence in human papillomavirus-transformed cervical cancer cells by targeting E6-AP ubiquitin ligase.
Sanduja S, Kaza V, Dixon DA. Sanduja S, et al. Aging (Albany NY). 2009 Sep 10;1(9):803-17. doi: 10.18632/aging.100086. Aging (Albany NY). 2009. PMID: 20157568 Free PMC article. - The dual role of sirtuins in cancer.
Bosch-Presegué L, Vaquero A. Bosch-Presegué L, et al. Genes Cancer. 2011 Jun;2(6):648-62. doi: 10.1177/1947601911417862. Genes Cancer. 2011. PMID: 21941620 Free PMC article. - Oxidative Stress Increases the Number of Stress Granules in Senescent Cells and Triggers a Rapid Decrease in p21waf1/cip1 Translation.
Lian XJ, Gallouzi IE. Lian XJ, et al. J Biol Chem. 2009 Mar 27;284(13):8877-87. doi: 10.1074/jbc.M806372200. Epub 2009 Jan 28. J Biol Chem. 2009. PMID: 19176530 Free PMC article.
References
- Blaxall B C, Dwyer-Nield L D, Bauer A K, Bohlmeyer T J, Malkinson A M, Port J D. Differential expression and localization of the mRNA binding proteins, AU-rich element mRNA binding protein (AUF1) and Hu antigen R (HuR), in neoplastic lung tissue. Mol Carcinog. 2000;28:76–83. - PubMed
- Buzby J S, Lee S M, Van Winkle P, DeMaria C T, Brewer G, Cairo M S. Increased GM-CSF mRNA stability in cord vs. adult mononuclear cells is translation dependent and associated with increased levels of A+U-rich element binding factor. Blood. 1996;88:2889–2897. - PubMed
- Campisi J. The biology of replicative senescence. Eur J Cancer. 1997;33:703–709. - PubMed
- Cristofalo V J, Pignolo R J, Cianciarulo F L, DiPaolo B R, Rotenberg M O. Changes in gene expression during senescence in culture. Exp Gerontol. 1992;27:429–432. - PubMed
- Cristofalo V J, Volker C, Francis M K, Tresini M. Age-dependent modifications of gene expression in human fibroblasts. Crit Rev Eukaryot Gene Expr. 1998;8:43–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous