Histone deacetylase 4 possesses intrinsic nuclear import and export signals - PubMed (original) (raw)
Histone deacetylase 4 possesses intrinsic nuclear import and export signals
A H Wang et al. Mol Cell Biol. 2001 Sep.
Abstract
Nucleocytoplasmic trafficking of histone deacetylase 4 (HDAC4) plays an important role in regulating its function, and binding of 14-3-3 proteins is necessary for its cytoplasmic retention. Here, we report the identification of nuclear import and export sequences of HDAC4. While its N-terminal 118 residues modulate the nuclear localization, residues 244 to 279 constitute an authentic, strong nuclear localization signal. Mutational analysis of this signal revealed that three arginine-lysine clusters are necessary for its nuclear import activity. As for nuclear export, leucine-rich sequences located in the middle part of HDAC4 do not function as nuclear export signals. By contrast, a hydrophobic motif (MXXLXVXV) located at the C-terminal end serves as a nuclear export signal that is necessary for cytoplasmic retention of HDAC4. This motif is required for CRM1-mediated nuclear export of HDAC4. Furthermore, binding of 14-3-3 proteins promotes cytoplasmic localization of HDAC4 by both inhibiting its nuclear import and stimulating its nuclear export. Unlike wild-type HDAC4, a point mutant with abrogated MEF2-binding ability remains cytoplasmic upon exogenous expression of MEF2C, supporting the notion that direct MEF2 binding targets HDAC4 to the nucleus. Therefore, HDAC4 possesses intrinsic nuclear import and export signals for its dynamic nucleocytoplasmic shuttling, and association with 14-3-3 and MEF2 proteins affects such shuttling and thus directs HDAC4 to the cytoplasm and the nucleus, respectively.
Figures
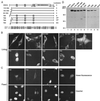
FIG. 1
Role of the N-terminal 118 residues of HDAC4 in regulating its nuclear localization. (A) Schematic illustration of HDAC4 and mutants. For HDAC4, 14-3-3 binding sites (S246, S467, and S632) and the Hda1-homology domain are depicted by boxes. In the triple mutants TM1 to TM4, the three serine residues critical for 14-3-3 binding are changed to alanine. Subcellular localization of HDAC4 and mutants is summarized at right: C, predominantly cytoplasmic; N, predominantly nuclear; P, pancellular. Shown at the lower part of the panel is the sequence comparison between homologous regions of HDAC4 (residues 90 to 142) and MAG1 (residues 504 to 556), with identical or conserved residues shaded. (B and C) Representative green fluorescence images of living (B) or fixed (C) NIH 3T3 cells expressing GFP or its fusion proteins. Cells were transfected with expression plasmids for GFP or its fusion proteins and subsequently analyzed by fluorescence microscopy 16 h after transfection. (B) Living cells were directly used for microscopic analysis. (C) Transfected cells were fixed with formaldehyde, counterstained with Hoechst 33528 and analyzed by green fluorescence microscopy (top), with corresponding Hoechst fluorescence images also taken (bottom). (D) Expression of GFP fusion proteins. 293 cells were transfected with expression plasmids for indicated GFP fusion proteins, and total cell extracts were analyzed by immunoblotting with anti-GFP antibody.
FIG. 2
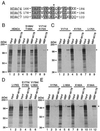
Mapping the dimerization domain of HDAC4. (A) Schematic representation of HDAC4 and deletion mutants. Motifs or domains are depicted by boxes as in Fig. 1A. (B to D) Interaction among HDAC4 proteins. HDAC4 deletion mutants were expressed as MBP fusion proteins in E. coli, immobilized on amylose agarose and incubated with HDAC4 or deletion mutants synthesized in vitro in the presence of
l
-[35S]methionine. Agarose beads were washed three times with buffer B–0.15 M KCl and once with buffer B–0.5 M KCl. Bound proteins were separated by SDS-PAGE and subsequently detected by autoradiography. Input represents 20% of the 35S-labeled protein used for each binding assay. Migrating positions of molecular markers are shown at the left of each panel.
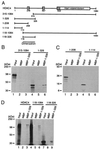
FIG. 3
Mapping the NLS of HDAC4. (A) Schematic representation of HDAC4 and deletion mutants. Motifs or domains are depicted by boxes as in Fig. 1A. Also indicated are two arginine-lysine-rich regions: RK1 (residues 132 to 184) and RK2 (residues 242 to 283). (B) Representative green fluorescence images of living cells expressing HDAC4 mutants fused to GFP. NIH 3T3 cells were transfected with expression plasmids for indicated GFP fusion proteins and analyzed by live green fluorescence microscopy. (C) Expression of GFP fusion proteins. 293 cells were transfected with expression plasmids for indicated GFP fusion proteins, and total cell extracts were analyzed by immunoblotting with anti-GFP antibody.
FIG. 4
Mutational analysis of the NLS. (A) Illustration of mutant 206-326 and its point mutants. The amino acid sequence of residues 242 to 283 of HDAC4 is listed, with potentially important arginine-lysine residues shown in boldface type. Residues important for 14-3-3 binding are labeled with asterisks. The point mutants PM1 to PM4 were derived from the deletion mutant 206-326 by substitution of indicated arginine-lysine residues. (B) Representative green fluorescence images of living NIH 3T3 cells expressing PM1 to PM4 fused to GFP. Cells were transfected with expression plasmids for indicated GFP fusion proteins, and green fluorescence microscopy was performed with living cells. For each mutant, two images are shown to illustrate distinct localization in different cells. (C) Expression of GFP fusion proteins. 293 cells were transfected with expression plasmids for indicated GFP fusion proteins, and cell extracts were analyzed by immunoblotting with anti-GFP antibody.
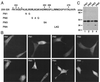
FIG. 5
Nuclear export activity of leucine-rich sequences of HDAC4. (A) Amino acid sequence of leucine-rich motifs of HDAC4, with leucine and methionine residues shown in boldface type. The consensus sequence of known leucine-rich export signals is also shown, with X denoting any amino acid residue. (B) Representative green fluorescence images of living cells expressing mutant 315-488 and its point mutant fused to GFP. NIH 3T3 cells were transfected with expression plasmids for the mutants, and green fluorescence microscopy was performed with living cells. (C) Expression of GFP fusion proteins. 293 cells were transfected with expression plasmids for GFP-315-488 and -315-488/S467A, and total cell extracts were analyzed by immunoblotting with anti-GFP antibody.
FIG. 6
Mapping intrinsic NES of HDAC4. (A) Schematic illustration of HDAC4 and its deletion mutants. Motifs or domains are depicted by boxes as in Fig. 1A. Subcellular localization of HDAC4 and mutants is summarized at right. (B and E) Representative green fluorescence images of living cells expressing HDAC4 and its deletion mutants fused to GFP. NIH 3T3 cells were transfected with expression plasmids for indicated GFP fusion proteins, and green fluorescence microscopy was performed with living cells. (C and F) Expression of GFP fusion proteins. 293 cells were transfected with expression plasmids for indicated GFP fusion proteins, and total cell extracts were analyzed by immunoblotting with anti-GFP antibody. (D and G) Effect of LMB on subcellular distribution of indicated GFP fusion proteins expressed in NIH 3T3 cells. After initial examination for green fluorescence, living cells expressing the indicated fusion proteins were treated with LMB (10 ng/ml), and their green fluorescence images were taken at indicated times. Under similar conditions, LMB had minimal effects on subcellular localization of GFP itself (data not shown).
FIG. 7
Mutational analysis of the NES. (A) Amino acid sequences of residues 1056 to 1069 of HDAC4 and mutants. M1059, L1062, V1064, and V1066 of HDAC4 are labeled with asterisks. For the point mutants, substituted and unchanged residues are indicated by the letter A (for alanine) and hyphens, respectively. (B) Representative green fluorescence images of living cells expressing HDAC4 mutants fused to GFP. NIH 3T3 cells were transfected with expression plasmids for indicated GFP fusion proteins, and green fluorescence microscopy was performed with living cells. (C) Expression of GFP fusion proteins. 293 cells were transfected with expression plasmids for indicated GFP fusion proteins, and total cell extracts were analyzed by immunoblotting with anti-GFP antibody. The asterisk at right marks the expected migrating position.
FIG. 8
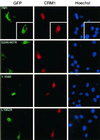
Effects of overexpressed CRM1 on subcellular localization of HDAC4 mutants. An HA-CRM1 expression plasmid was transfected into NIH 3T3 cells along with mammalian expression plasmids for HDAC4 mutants fused to GFP. At 16 h after transfection, cells were fixed and stained with antihemagglutinin antibody to detect exogenous CRM1 (middle, red) by indirect immunofluorescence microscopy. Green fluorescence was used to determine subcellular localization of GFP fusion proteins (left) (green). The cells were counterstained with Hoechst 33528 to visualize nuclei (right) (blue). While endogenous CRM1 is enriched around the nuclear envelope, overexpressed CRM1 has been found to be pancellular or nuclear (17, 71).
FIG. 9
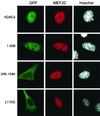
Effects of exogenous MEF2C on nuclear localization of HDAC4 and mutants. The MEF2C expression plasmid was transfected into NIH 3T3 cells along with mammalian expression plasmids for GFP fusion proteins of HDAC4 or its mutants. At 16 h after transfection, cells were fixed and stained with anti-MEF2C antibody to detect MEF2C by indirect immunofluorescence microscopy (middle) (red). Green fluorescence was used to determine subcellular distribution of GFP fusion proteins (left) (green). The cells were counterstained with Hoechst 33528 to visualize nuclei (right) (white).
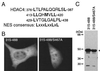
FIG. 10
Mutational analysis of the MEF2-binding site of HDAC4. (A) Sequence comparison of residues 166 to 184 of HDAC4 with the corresponding regions of HDAC5 and HDAC7. Identical or conserved residues are shaded, and L175 of HDAC4 is indicated by an asterisk. (B to E) Interaction of the MEF2C mutant M178 with HDAC4 and point mutants. MBP or MBP-M178 was immobilized on amylose agarose and tested for interaction with HDAC4 or mutants synthesized in vitro in the presence of [35S]methionine. Bound proteins were separated by SDS-PAGE and subsequent autoradiography. Input represents 20% of the 35S-labeled protein used for each binding assay. Migrating positions of molecular markers are shown at the left of each panel, whereas the positions of HDAC4 and its mutants are indicated by asterisks at right.
FIG. 11
(A) Model depicting how subcellular localization of HDAC4 is controlled. HDAC4 possesses intrinsic nuclear import and export signals important for its dynamic nucleocytoplasmic shuttling. Association with 14-3-3 or MFE2 proteins modulates the shuttling. 14-3-3 binding promotes cytoplasmic localization of HDAC4 by both inhibiting its nuclear import and stimulating its nuclear export, whereas MEF2 interacts with HDAC4 and targets it to the nucleus. While phosphorylation of S246, S467, and S632 of HDAC4 stimulates the binding of 14-3-3 proteins, it remains less clear how the interaction between MEF2 and HDAC4 is regulated. (B) Sequence comparison of the NLS of HDAC4 with the corresponding regions of HDAC5, MITR and HDAC7. Critical residues of the HDAC4 NLS are boxed with related residues of the other three proteins. (C) Sequence alignment of the NES of HDAC4 with the related regions of HDAC5 and HDAC7. Critical residues of the HDAC4 NES are boxed with corresponding residues of HDAC5 and HDAC7.
Similar articles
- The nuclear localization domain of the MEF2 family of transcription factors shows member-specific features and mediates the nuclear import of histone deacetylase 4.
Borghi S, Molinari S, Razzini G, Parise F, Battini R, Ferrari S. Borghi S, et al. J Cell Sci. 2001 Dec;114(Pt 24):4477-83. doi: 10.1242/jcs.114.24.4477. J Cell Sci. 2001. PMID: 11792813 - HDAC4 deacetylase associates with and represses the MEF2 transcription factor.
Miska EA, Karlsson C, Langley E, Nielsen SJ, Pines J, Kouzarides T. Miska EA, et al. EMBO J. 1999 Sep 15;18(18):5099-107. doi: 10.1093/emboj/18.18.5099. EMBO J. 1999. PMID: 10487761 Free PMC article. - HDAC4, a human histone deacetylase related to yeast HDA1, is a transcriptional corepressor.
Wang AH, Bertos NR, Vezmar M, Pelletier N, Crosato M, Heng HH, Th'ng J, Han J, Yang XJ. Wang AH, et al. Mol Cell Biol. 1999 Nov;19(11):7816-27. doi: 10.1128/MCB.19.11.7816. Mol Cell Biol. 1999. PMID: 10523670 Free PMC article. - Caspase-dependent regulation of histone deacetylase 4 nuclear-cytoplasmic shuttling promotes apoptosis.
Paroni G, Mizzau M, Henderson C, Del Sal G, Schneider C, Brancolini C. Paroni G, et al. Mol Biol Cell. 2004 Jun;15(6):2804-18. doi: 10.1091/mbc.e03-08-0624. Epub 2004 Apr 9. Mol Biol Cell. 2004. PMID: 15075374 Free PMC article. - Differential localization of HDAC4 orchestrates muscle differentiation.
Miska EA, Langley E, Wolf D, Karlsson C, Pines J, Kouzarides T. Miska EA, et al. Nucleic Acids Res. 2001 Aug 15;29(16):3439-47. doi: 10.1093/nar/29.16.3439. Nucleic Acids Res. 2001. PMID: 11504882 Free PMC article.
Cited by
- Nuclear calcium signaling regulates nuclear export of a subset of class IIa histone deacetylases following synaptic activity.
Schlumm F, Mauceri D, Freitag HE, Bading H. Schlumm F, et al. J Biol Chem. 2013 Mar 22;288(12):8074-8084. doi: 10.1074/jbc.M112.432773. Epub 2013 Jan 30. J Biol Chem. 2013. PMID: 23364788 Free PMC article. - Microtubule-dependent subcellular redistribution of the transcriptional coactivator p/CIP.
Qutob MS, Bhattacharjee RN, Pollari E, Yee SP, Torchia J. Qutob MS, et al. Mol Cell Biol. 2002 Sep;22(18):6611-26. doi: 10.1128/MCB.22.18.6611-6626.2002. Mol Cell Biol. 2002. PMID: 12192059 Free PMC article. - Tumor necrosis factor alpha-mediated reduction of KLF2 is due to inhibition of MEF2 by NF-kappaB and histone deacetylases.
Kumar A, Lin Z, SenBanerjee S, Jain MK. Kumar A, et al. Mol Cell Biol. 2005 Jul;25(14):5893-903. doi: 10.1128/MCB.25.14.5893-5903.2005. Mol Cell Biol. 2005. PMID: 15988006 Free PMC article. - HDAC inhibitors and neurodegeneration: at the edge between protection and damage.
Dietz KC, Casaccia P. Dietz KC, et al. Pharmacol Res. 2010 Jul;62(1):11-7. doi: 10.1016/j.phrs.2010.01.011. Epub 2010 Feb 1. Pharmacol Res. 2010. PMID: 20123018 Free PMC article. Review. - Histone Deacetylase 4 Downregulation Elicits Post-Traumatic Psychiatric Disorders through Impairment of Neurogenesis.
Saha P, Gupta R, Sen T, Sen N. Saha P, et al. J Neurotrauma. 2019 Dec 1;36(23):3284-3296. doi: 10.1089/neu.2019.6373. Epub 2019 Aug 1. J Neurotrauma. 2019. PMID: 31169064 Free PMC article.
References
- Brown C E, Lechner T, Howe L, Workman J L. The many HATs of transcription coactivators. Trends Biochem Sci. 2000;25:15–19. - PubMed
- Champagne N, Bertos N R, Pelletier N, Wang A H, Vezmar M, Yang Y, Heng H H, Yang X J. Identification of a human histone acetyltransferase related to monocytic leukemia zinc finger protein. J Biol Chem. 1999;274:28528–28536. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials